
Calculus: Early Transcendentals
8th Edition
ISBN: 9781285741550
Author: James Stewart
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Pavnm.m.
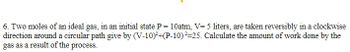
Transcribed Image Text:6. Two moles of an ideal gas, in an initial state P = 10atm, V=5 liters, are taken reversibly in a clockwise
direction around a circular path give by (V-10)²+(P-10)²=25. Calculate the amount of work done by the
gas as a result of the process.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Similar questions
- PARTICIPATION 3.3.3: Ratios. АCTIVITY 1) A daycare center expects 20 babies on a given day. How many staff should the center have work that day to maintain a 1/4 ratio? Check Show answer 2) An adult soccer league requires a ratio of at least 2 women per 7 men on the roster. If 14 men are on the roster, how many women are needed to maintain that ratio? Check Show answer 3) A lemonade recipe requires 1 cup sugar per 8 cups water, for a 1/8 ratio. Andy's daughter is helping and accidentally puts 2 cups of sugar into the pitcher. "No problem," says Andy, "we'll just add more water." How many total cups of water should be put in the pitcher to maintain the 1/8 ratio? Check Show answerarrow_forward6 of 36 Text-to-Speech 5. If the radius, 8 cm, of a cone is increased by 120% of it's original value, but its height, 6 cm, doesn't change, by approximately how many cubic centimeters does the volume of the cone increase? 3 A. 1543 cm 3 B. 402 cm C. 1945 cm3 3 D. 17.6 cm Powered by Linklt! 山T FEB tv ....arrow_forwardSIC Code No. Emp. No. Prod. Wkrs. Value Added by Mfg. Cost of Materials Value of Indus. Shipmnts New Cap. Exp. End Yr. Inven. Indus. Grp. 201 433 370 23518 78713 4 1833 3630 1 202 131 83 15724 42774 4 1056 3157 1 203 204 169 24506 27222 4 1405 8732 1 204 100 70 21667 37040 4 1912 3407 1 205 220 137 20712 12030 4 1006 1155 1 206 89 69 12640 13674 3 873 3613 1 207 26 18 4258 19130 3 487 1946 1 208 143 72 35210 33521 4 2011 7199 1 209 171 126 20548 19612 4 1135 3135 1 211 21 15 23442 5557 3 605 5506 2 212 3 2 287 163 1 2 42 2 213 2 2 1508 314 1 15 155 2 214 6 4 624 2622 1 27 554 2 221 52 47 2471 4219 2 292 929 3 222 74 63 4307 5357 2 454 1427 3 223 13 12 673 1061 1 20 325 3 224 17 13 817 707 1 84 267 3 225 169 147 8986 10421 3 534 2083 3 226 51 41 3145 4140 2 220 697 3 227 55 44 4076 7125 2 176 1446 3 228 84 76 3806 8994 2 423 1014 3 229 61 47 4276 5504 2 464 1291 3 231 27 22 1239 716 1 22 356 4 232 200 178 9423 8926 3 200 2314 4 233 294…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Calculus: Early TranscendentalsCalculusISBN:9781285741550Author:James StewartPublisher:Cengage Learning
Calculus: Early TranscendentalsCalculusISBN:9781285741550Author:James StewartPublisher:Cengage Learning Thomas' Calculus (14th Edition)CalculusISBN:9780134438986Author:Joel R. Hass, Christopher E. Heil, Maurice D. WeirPublisher:PEARSON
Thomas' Calculus (14th Edition)CalculusISBN:9780134438986Author:Joel R. Hass, Christopher E. Heil, Maurice D. WeirPublisher:PEARSON Calculus: Early Transcendentals (3rd Edition)CalculusISBN:9780134763644Author:William L. Briggs, Lyle Cochran, Bernard Gillett, Eric SchulzPublisher:PEARSON
Calculus: Early Transcendentals (3rd Edition)CalculusISBN:9780134763644Author:William L. Briggs, Lyle Cochran, Bernard Gillett, Eric SchulzPublisher:PEARSON Calculus: Early TranscendentalsCalculusISBN:9781319050740Author:Jon Rogawski, Colin Adams, Robert FranzosaPublisher:W. H. Freeman
Calculus: Early TranscendentalsCalculusISBN:9781319050740Author:Jon Rogawski, Colin Adams, Robert FranzosaPublisher:W. H. Freeman
 Calculus: Early Transcendental FunctionsCalculusISBN:9781337552516Author:Ron Larson, Bruce H. EdwardsPublisher:Cengage Learning
Calculus: Early Transcendental FunctionsCalculusISBN:9781337552516Author:Ron Larson, Bruce H. EdwardsPublisher:Cengage Learning

Calculus: Early Transcendentals
Calculus
ISBN:9781285741550
Author:James Stewart
Publisher:Cengage Learning

Thomas' Calculus (14th Edition)
Calculus
ISBN:9780134438986
Author:Joel R. Hass, Christopher E. Heil, Maurice D. Weir
Publisher:PEARSON

Calculus: Early Transcendentals (3rd Edition)
Calculus
ISBN:9780134763644
Author:William L. Briggs, Lyle Cochran, Bernard Gillett, Eric Schulz
Publisher:PEARSON

Calculus: Early Transcendentals
Calculus
ISBN:9781319050740
Author:Jon Rogawski, Colin Adams, Robert Franzosa
Publisher:W. H. Freeman


Calculus: Early Transcendental Functions
Calculus
ISBN:9781337552516
Author:Ron Larson, Bruce H. Edwards
Publisher:Cengage Learning