
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
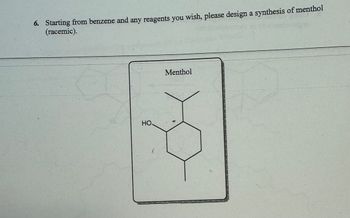
Transcribed Image Text:6. Starting from benzene and any reagents you wish, please design a synthesis of menthol
(racemic).
HO.
L
Menthol
MATE
NONCALLIEFIED
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- C. 1.) For each reaction, determine the best set of reagents to use to perform the transformation. b. a. Br OH I OH OHarrow_forwardAnswer each question thoroughly nd completely. 1. Thoroughly explain why a halogen, X2 ,cannot be directly added to a benzene ring. 2. What properties does a benzene ring have that allude to its reactivity? 3. What is meant by aliphatic? 4. Explain the different types of oxidations for phenols. Give an example of each with the name of the phenol, and the oxidized phenol product. The examples should be drawn in chemical reaction form. 5. Are phenols reactive? Why or why not? 6. What is meant by an ortho director? Use a reaction as an example. 7. Why is the rate limiting step important in the bromination of benzene? 8. What role does the sigma complex play in electrophilic aromatic substitution reactions? 9. How do Lewis acids play a role in generation of the electrophile? A mechanism will be needed to go along with your explanation. 10. Explain the nitration of benzene reaction. 11. What are deactivating groups, and how do they relate to substitution on the benzene ring? 12. What is the…arrow_forward1. Fill in the necessary starting material, reactants or product for the reactions shown below. A. B. C. I. NaNH,, NH, 2. CH₂CH₂CH₂CH₂Cl 1. NaNH,, NH, 2. CH₂CH₂CI 2. Show the complete mechanism for A. and B. from above. Use the back if necessary.arrow_forward
- Identify the starting materials for these target molecules. Only go back one step in the reaction – meaning cleave the target molecule and translate those - synthons into synthetic equivalents. a. b. N N Target Molecule Ph Target Moleculearrow_forward5. Propose a synthesis of each of the following compounds using the indicated starting material. You may use any organic compounds, inorganic compounds, organometallic compounds, or solvents of your choice. Do not show any reactive intermediates, mechanisms, or transition states, but be sure to show each isolable compound along your synthetic route. a. b. C. Ph + PPh3 Cl mylom Ph. CH3 steps steps steps H3C. H3C Ph CH3 CH3 Ph OH ÕH Ph (racemic) 4arrow_forwardNeed help with Part 2arrow_forward
- Choose the right answerarrow_forward3. Draw the carbocation intermediate that the halide must attack to form the given product for each of the reactions below. a. b. ن d. Br Br Br Br 2 پر مشوarrow_forwardDevise a synthesis of each compound from benzene. You may use any other organic or in organic reagents.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY