
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I need help with this
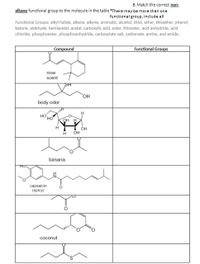
Transcribed Image Text:6. Match the correct non-
alkane functional group to the molecule in the table "There may be more than one
functional group, include all
Functional Groups: alkyl halide, alkene, alkyne, aromatic, alcohol, thiol, ether, thioether, phenol,
ketone, aldehyde, hemiacetal, acetal, carboxylic acid, ester, thioester, acid anhydride, acid
chloride, phosphoester, phosphoanhydride, carboxylate salt, carbonate, amine, and amide.
Compound
Functional Groups
rose
scent
HO.
body odor
HO
HO
OH H
OH
он
ist
banana
capsaicin
(spicy)
coconut
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What functional groups are present in THC-O? Please circle on the chemical structure that is given.arrow_forwardReplace one H on propane with Br. Does it matter which H is replaced? Yes or No (circle) Are isomers possible for C3H¬B1? Yes or No (circle) Draw the expanded structures for all isomers and name each compound. As in question 1 on the previous page, use a number in front of the name to indicate the position for the bromine atom on the carbon chain. Consult with your instructor to make sure you've correctly drawn and named the structure(s) here. 3. Complete the following for compounds derived from butane, C4H10. Butane, C4H10 Lewis Structures & Name Are isomers possible? Yes or No (circle) Build a model & draw the line structures for all potential structural isomers of C4H10. Using your molecular models for the butane isomers as a guide, draw line structures for all monoiodo-derivatives of each of your isomers of butane having the molecular formula C4H9I. Give the IUPAC name for each compound.arrow_forwardcould you identify functional group? the molecualr formula for this is C9H10O3 and DBE is 5arrow_forward
- Question 5 Please answer everything clearly and correctly, thanks! Like will be given!arrow_forward1. FUNdamentals a) Name the following functional groups. Put the name of each functional group into the box. ОН SHarrow_forward2 O L 40% A moodle.lsua.edu Alanie Fontenot my.LSUA HEM 1001/LEC/O01X-2021/SPRING/SEM - ro Chemistry for Non-Science Major I e / My courses / 2021 | 2021/SPRING / CHEM 1001/LEC/001X-2021/SPRING/SEM / Chapter 4 Functional Groups estion 3 What is the functional group found in the following molecule? t yet H H. swered pints out of H -C-C C - Flag uestion 0-C- C-H Select one: a. amide O b. carboxylic acid O c. ester O d. ether O e. ketone HIU- I- ООО О Оarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY