
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working
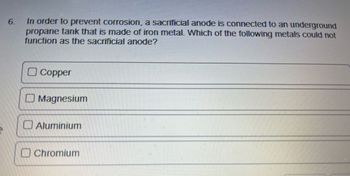
Transcribed Image Text:6.
In order to prevent corrosion, a sacrificial anode is connected to an underground
propane tank that is made of iron metal. Which of the following metals could not
function as the sacrificial anode?
Copper
Magnesium
Aluminium
Chromium
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Hello, I hope you are doing well on this fine day. For the following quetion please read carefully the question and instruction. PLEASE ANSWER QUESTION IN 20 MINTUES NOT MORE PLEASE AND THANK YOU. If you do answer the question correctly and post it in the next 20 minutes, NO NEED TO SHOW ALL THE WORK, I JUST WOULD LIKE THE CORRECT ANSWER AS SOON AS POSSIBLE. I will write a wonderful and generous feedback/review/rating about you.arrow_forwardem 119 My Course X Macmillan: X Course Mo Submit Answer X Sections 5 What is the FORMULA for the limiting reagent? References X age.com/static/nb/ui/evo/index.html?deploymentid=5735112480241329813180832311&elSBN=9781305862883&id=1707786042&snapsh What amount of the excess reagent remains after the reaction is complete? HOMEWOR Use the References to access Important values if needed for this question. For the following reaction, 20.3 grams of carbon dioxide are allowed to react with 39.6 grams of potassium hydroxide. carbon dioxide (g) + potassium hydroxide (aq) potassium carbonate (aq) + water (1) - What is the maximum amount of potassium carbonate that can be formed? MacBook Air X grams MindTap - grams ;arrow_forwardFind the value of xarrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardsession.masteringchemistry.com/myct/itemView?assignment ProblemID=190556628&offset=next Discussion Question 1: Chapter 6 Homework blem 6.20- Enhanced - with Feedback Enter the symbols for the ions and the correct formula for the onic compound formed by each of the following. esc Q A N F1 2 W S F2 X #3 20 E D F3 с S4 $ OOO 000 R Part E F4 F Enter the symbols for the ions of sodium and phosphorus. Enter the lons formed by these elements and separate your answers with a comma (e.g., Sr²+, As³-). cation, anion = Part F Complete previous part(s) Submit Part G cation, anion Submit Part H Complete previous part(s) 65° V Enter the symbols for the ions of calcium and sulfur. Enter the lons formed by these elements and separate your answers with a comma % Request Answer T Request Answer G 6 ΑΣΦ B ΑΣΦ MacBook Air F6 Y H 18⁰ h & 7 N F7 U * CO 8 J BUT RAS poddany ▶II ? F8 1 ? E 9 K F9 O L (e.g., Sr²+, As³-). 0 Ga3+ and 02-E L F10 P F11arrow_forwardEnter the ions present in a solution of K₂CO3. Express your answers as chemical formulas separated by a comma. Offset subscripts and charges on each ion; for charges, write the number before the + or - sign. View Available Hint(s) 5 Provide Feedback Submit esc ΑΣΦ Mother to Son &....pdf A lock ! 1 F1 Q GOOD A @ 2 N 30 F2 W S ? #3 80 F3 X E I дв control option command D A SA 4 Q F4 C R FL O % 5 & F5 T V MacBook Air 6 G F6 Y B & 7 H F7 U N * 8 J DII FB 1 ( 9 M K FO O ) V H 0 < L F10 P commandarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY