
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
A. Draw the most likely structure of each product in the boxes attached below.
B. What mechanism is responsible for making the product at 2.82 minutes?
C. What mechanism is responsible for making the product at 3.81 minutes?
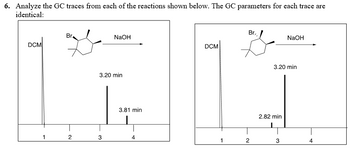
Transcribed Image Text:6. Analyze the GC traces from each of the reactions shown below. The GC parameters for each trace are
identical:
Br.
NaOH
DCM
3.20 min
3.81 min
Br,
NaOH
DCM
1
2
4
1
2
3.20 min
2.82 min
Transcribed Image Text:2.82 min
3.20 min
3.81 min
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 3. Calculate the mass of AgCl that can be dissolved in 10 mL of water. The Ksp for Silver Chloride is: 1.83 x 10-10. Group of answer choices a, 1.94e5 b, 2.58e5 c, 2.58e-5 d, 1.94e-5arrow_forwardWrite the expression for the K₁ for each of the following reactions HCO3¹1+ H₂O OH-1 + H₂CO3 -1 [ ][ Kb = [ ] HPO4-² + H₂O OH-¹ + H₂PO4¹ ][ Kb = [ ] c. phosphoric a. acetic b. hydrochloric g. sulfurous h. hydrosulfuric n. HPO4² 0. H₂PO4-1 p. CO3-² i. OH-1 -2 -3 u. H2O v. H₂CO3 W. PO4³ d. perchloric j. H30+1 q. HCO3-1 r. H₂S k. SO4² e. hydrofluoric 1. Mg+2 S. HS-1 t. S-² f. sulfuric m. Cl-¹arrow_forwarded 20 A student measures the Pb2+ concentration in a saturated aqueous solution of lead chloride to be 1.66x10-2 M. Based on her data, the solubility product constant for lead chloride is E 5 F3 1 Submit Answer C $ 4 000 DOD F4 R F V % 5 Retry Entire Group 9 more group attempts remaining T Cengage Learning Cengage Technical Support FS G ^ 6 B MacBook Air Fo Y H & 7 N F7 U J * 8 ►ll M F8 1 ( 9 K F9 O V H 1 ) 0 L command F10 P B - > : 4) ; F11 alt { Previous [ option + = ? Next> 1 Save and Exit 7 F12 } 1 delete enter return 8 starrow_forward
- 9-arrow_forwardPAP Chemistr x G diagram show X PAP Chemistr x G Reactions that X EPAP Chemistr X PAP Chemistr X HAC Session Timer X Mp Rocking Whee X + b ar-2903012.agilixbuzz.com/student/135113422/activity/c412d902-b23e-4202-b813-76alf9f4c196 175% Reset Mastery Assess It_8 Enrique Solis PAP Chemistry-2903012-42100P-1... All changes saved 6. You have a very concentrated solution (12 M) of potassium chloride (KCI). You need it to be at the lowest concentration possible for the experiment you are about to conduct. The problem is you forgot to order lab supplies, so you only have 2 L of distilled water left. What would be the final concentration if you added the two 2 L of distilled water to the 0.5 L of 12 M KCI? 3.0 M 2.4 M 24 M 48 Marrow_forwardKk.364.arrow_forward
- For 2SO2 (g) + O2 (g) <-------> 2SO3 (g) Concentrations: [SO2]=0.20, [O2]=0.80, [SO3]= 0.43. What is Kc for the reaction?arrow_forward1. Write the dissociation reaction and solubility product constant expression for the following sparingly soluble salt. a. AgBr Ksp = 5.2 x 10-13 b. PbCl2 Ksp = 1.7 x 10-5 %3D 2. Determine the Molar Solubility of the following sparingly soluble salt in water. a. AgBr Ksp = 5.2 x 10-13 b. PBCI2 Ksp = 1.7 x 10-5 3. Express the solubility (S) in question #2 in g/L.arrow_forwardE PAP Chem G diagram sh x H PAP Chemi x G Reactions t x K PAP Chemi: x I PAP Chemi x b ar-2903012.agilixbuzz.com/student/135113422/activity/c412d902-b23e-4202-b813-76alf9f4c196 I Student Ap x Session Tir X Mp Rocking WX Mastery Assess It_8 200% + Reset PAP Chemistry-2903012-42100. Enrique Solis All changes saved 3. Reactions that occur in the gaseous state are affected by changing the volume of the container. Which choice best describes what will happen to the reaction if the volume of the container is decreased? The reaction will speed up. The reaction will shift left. The reaction will not change. SAVE & EXIT NEXT The reaction will shift right. 3 of 25 PREVIOUSarrow_forward
- Review the reversible reactions given, along with the associated equilibrium constant K at room temperature. In each case, determine whether the forward or reverse reaction is favored. AgCl → Ag* + Cl'Ksp=1.6 x 1010 Choose... - A + B + CK=4.9 x 103 Choose... CH3COOH + CH3C00" + H*Kg=1.8 x 10-5 Choose... Alš+ + 30H Ksp=3.7 x 1015 Choose... - Choose... Reverse Forward Al(OH)3arrow_forwardMISSED THIS? Read Section 16.7 (Pages 699-701): Watch KCV 16.Z WE 16.7. Silver sulfate dissolves in water according to the reaction Ag-SO. (8)=2Ag (aq) +SO.² (aq) K. = 1.1 x 10-5 at 298 K A2.50-L, solution contains 10.9 g of dissolved silver sulfate If additional solid silver sulfate is added to the solution, will it dissolve? Match the words in the left column to the appropriate blanks in the sentences on the right. saturated equal to supersaturated Submit less than will not greater than will unsaturated Request Answer Since Qis Reset Help K. the solution is Thus, when more solid is added it dissolve.arrow_forwardThe Ksp for SrF2 is 2.45 x 10-9. Use this information to calculate the solubility of Strontium Fluoride in water (find the [Sr2+] and [F-] at equilibrium). SrF2 (s) Sr2+ (aq) + 2F- (aq) I C Earrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning