
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
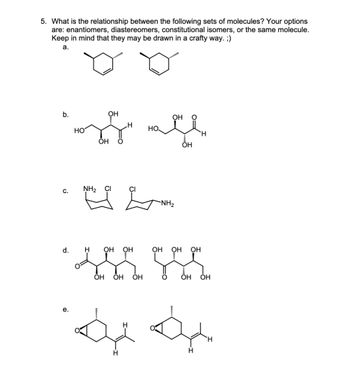
Transcribed Image Text:5. What is the relationship between the following sets of molecules? Your options
are: enantiomers, diastereomers, constitutional isomers, or the same molecule.
Keep in mind that they may be drawn in a crafty way. ;)
a.
b.
ОН
позади посе
H
OH
с.
NH₂
e.
CI
******
d. H ОН
H
за
ОН OH ОН
CI
OH
H
-NH₂
ОН ОН
ОН
OH ОН
H
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- T. Please dont provide the handwriting solution.arrow_forwardHow are the following molecules related? J. OH constitutional isomers completely different diastereomers exactly the same O enantiomers .... CI Moving to another question will save this response.arrow_forward4. The following molecules are represented without stereochemistry. Identify the chiral centers present in each molecule and draw all of the potential stereoisomers available. Avoid drawing duplicate structures. 5arrow_forward
- Suppose compounds A and B are enantiomers. What can be said about A and B (select all that apply). a. They are mirror images of eachother b. Neither contains a chiral center c. They consist of the same number & types of atoms d. They are superimposible e. They are NOT superimposable f. They are stereoisomersarrow_forwardRank the following structures in terms of conformational stability. If two or more are of equal stability, ndicate this. Justify your answer with chair conformations. B с Ꭰ |||arrow_forwardPlease don't provide handwritten solution ....arrow_forward
- Consider (2R,3R)-2,3-dioidobutane: (a)Draw a Fischer Projection of this structure with C1 on top and C4 on bottom. (b)Draw a Newman Projection of of the meso compound of 2,3-dibromobutane looking down the C2-C3 bondwith C2 in front, and label your asymmetric carbons as S or R.arrow_forwardSeveral compounds are shown below. For each, make two models: one model of the compound as shown, and the other where each tetrahedral stereocenter is inverted. Compare the two models side-by-side to see if the model with the inverted stereocenter(s) is the enantiomer, or if it is identical to the original. Remember, you can rotate around o-bonds to place the molecule in the conformation with the highest symmetry. Is each compound meso or not? OH OH OH OH DOKOOK OH OH Compound 1 Compound 2 Compound 1's mirror image is identical to Compound 1 Compound 1's mirror image is the enantiomer of Compound 1 Compound 1 is meso Compound 2's mirror image is identical to Compound 2 Compound 2's mirror image is the enantiomer of Compound 2 Compound 2 is meso Compound 3's mirror image is identical to Compound 3 Compound 3's mirror image is the enantiomer of Compound 3 Compound 3 is meso Compound 4's mirror image is identical to Compound 4 Compound 4's mirror image is the enantiomer of Compound 4…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY