
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
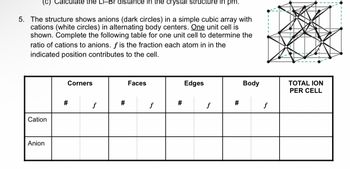
Transcribed Image Text:(c) Calculate the Li-Br distance in the crystal structure in pm.
5. The structure shows anions (dark circles) in a simple cubic array with
cations (white circles) in alternating body centers. One unit cell is
shown. Complete the following table for one unit cell to determine the
ratio of cations to anions. f is the fraction each atom in in the
indicated position contributes to the cell.
Cation
Anion
Corners
#
f
#
Faces
f
#
Edges
f
#
Body
TOTAL ION
PER CELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Outline a two-dimensional unit cell for the pattern shown here. If the black squares are labeled A and the white squares are B, what is the simplest formula for a compound based on this pattern?arrow_forwardIn the LiCl structure shown in Figure 9.21, the chloride ions form a face-centered cubic unit cell 0.513 nm on an edge. The ionic radius of Cl- is 0.181 nm. (a) Along a cell edge, how much space is between the Cl- ions? (b) Would an Na+ ion (r=0.095nm) fit into this space? a K+ ion (r=0.133nm)?arrow_forwardDescribe the crystal structure of Pt, which crystallizes with four equivalent metal atoms in a cubic unit cell.arrow_forward
- What is the relationship between the structures of buckminsterfullerene and carbon nanotubes?arrow_forward8.76 Using circles, draw regular two-dimensional arrangements that demonstrate low packing efficiency and high packing efficieny.arrow_forwardOutline a two-dimensional unit cell for the pattern shown here. If the black squares are labeled A and the white squares are B, what is the simplest formula for a compound based on this pattern?arrow_forward
- Explain in words how Avogadros number could be obtained from the unit-cell edge length of a cubic crystal. What other data are required?arrow_forwardCaTiO3, a perovskite, has the structure below. (a) If the density of the solid is 4.10 g/cm3, what is the length of a side of the unit cell? (b) Calculate the radius of the Ti4+ ion in the center of the unit cell. How well does your calculation agree with a literature value of 75 pm? Unit cell of the perovskite CaTiO3 A sample of perovskite, iO3 FIGURE 7.11 Relative sizes of some common ions. Rodii are given in picometers (1 pm 1 1012 m). (Data taken from J. Emsley, The Elements, Clarendon Press, Oxford, 1998, 3rd edition.)arrow_forwardAt 25 C, how high will water rise in a glass capillary tube with an inner diameter of 0.63 mm? Refer to Example 10.4 for the required information.arrow_forward
- A metal burns in air at 600c under high pressure to form an oxide with formula MO2. This compound is 23.72% oxygen by mass. The distance between the centers of touching atoms in a cubic closest packed crystal of this metal is 269.0 pm. What is this metal? What is its density?arrow_forward• describe the arrangement of atoms in the common cubic crystal lattices and calculate the packing efficiency for a lattice.arrow_forward8.97 The doping of semiconductors can be done with enough precision to tune the size of the band gap in the material. Generally, in order to have a larger band gap, the dopant should be smaller than the main material. If you are a materials engineer and need a semiconductor that has lower conductivity thin pure silicon, what clement or elements could you use as your dopant? (You do not want either an n- or a p- type material) Explain your reasoning.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning