
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
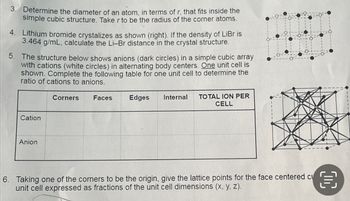
Transcribed Image Text:3. Determine the diameter of an atom, in terms of r, that fits inside the
simple cubic structure. Take r to be the radius of the corner atoms.
4. Lithium bromide crystalizes as shown (right). If the density of LiBr is
3.464 g/mL, calculate the Li-Br distance in the crystal structure.
5.
The structure below shows anions (dark circles) in a simple cubic array
with cations (white circles) in alternating body centers. One unit cell is
shown. Complete the following table for one unit cell to determine the
ratio of cations to anions.
Cation
Anion
Corners Faces
Edges Internal
TOTAL ION PER
CELL
6. Taking one of the corners to be the origin, give the lattice points for the face centered cu €
unit cell expressed as fractions of the unit cell dimensions (x, y, z).
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- The density of Ni is 8.90 g/cm3. Does nickel crystallize in a simple cubic structure? Explain.arrow_forwardBarium oxide (BaO] crystallizes in a structure in which the O² io are in a face-centered cubic lattice and the Ba²+ ions are in octahedral holes. What is the number of Ba²+ ions in the unit ce 2 V = a³ = (4.47x10-8cm)³ = 8.93x10-23 cm³ 4.72x10¯ 5.28 g cm formul 3 8 = 4.01 formula unitsarrow_forwardPlease explainarrow_forward
- I need help with these two questions?arrow_forwardonds remain. This test can only be taken once. Attempts Force Once started, this test must be completed in one sitting. Do not leave the test before clicking Save and Submit Completion Your answers are saved automatically. Remaining Time: 1 hour, 57 minutes, 02 seconds. * Question Completion Status: A Moving to another question will save this response. Question 15 Silver (r = 0.144 nm) crystallizes in a face centered cubic cell. What is the volume of the unit cell in cubic centimeters? What is the density of silver in g/cm3? Moving to another question will save this response.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY