
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
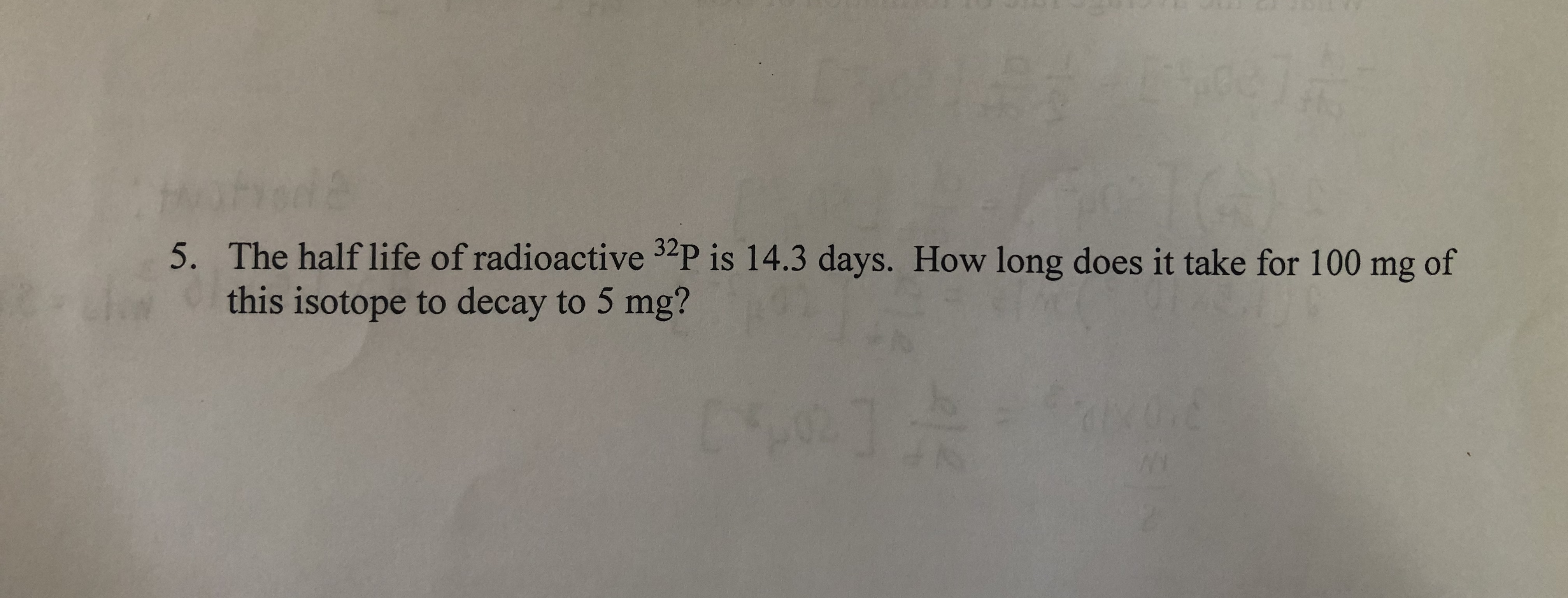
Transcribed Image Text:5. The half life of radioactive 32P is 14.3 days. How long does it take for 100 mg of
this isotope to decay to 5 mg?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- A radioactive isotope has a half life of 25.9 minutes. How long will it take for the radiation from a 580 pCi sample to decrease to 290 pCi?arrow_forward3. Suppose a sample of carbon-243 has a half-life of 3427 years. How long does it take for 80% of a sample of carbon-243 to decay?arrow_forwardTin-129 is radioactive and has a half life of 2.23 minutes. How long would it take a sample to decay from 8.50mg to 4.00mg .arrow_forward
- What is the half‑life of an isotope that decays to 3.125%3.125% of its original activity in 41.8 h?arrow_forwardHow many hours are needed for a 16.0 g sample of Si-31 to decay to 2.0 g? The half-life for Si-31 is 2.6 h.arrow_forwardRadioactive vanadium-48 decays with a half-life of 16.0 days. a. What is the value of k in s? k = b. A sample contains 84.0 mg *°V. How many decay events will be produced in the first second? Assume the atomic mass of **V is 48.0 u. decays/s c. A chemist obtains a fresh sample of 48 V and measures its radioactivity. She then determines that to do an experiment, the radioactivity cannot fall below 25% of the initial measured value. How long does she have to do the experiment? daysarrow_forward
- A radioactive sample has a decay constant of 1.32 x 10-3 h-1. What is its half life?arrow_forwardIt takes 6400 years for one gram of a radioactive element to decay away to only 1/16 of a gram. The half-life of this element isarrow_forward2. Tellurium-123 is a radioactive isotope occurring in natural tellurium. The decay constant is 1.7 x 1021 / s. What is the half-life in years?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY