
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Plutonium-236 is an alpha emitter with a half-life of 2.86 years. How long will it take for the 1.20 mg sample of Pu−236 to decay to 0.160 mg ?
Expert Solution
arrow_forward
Step 1
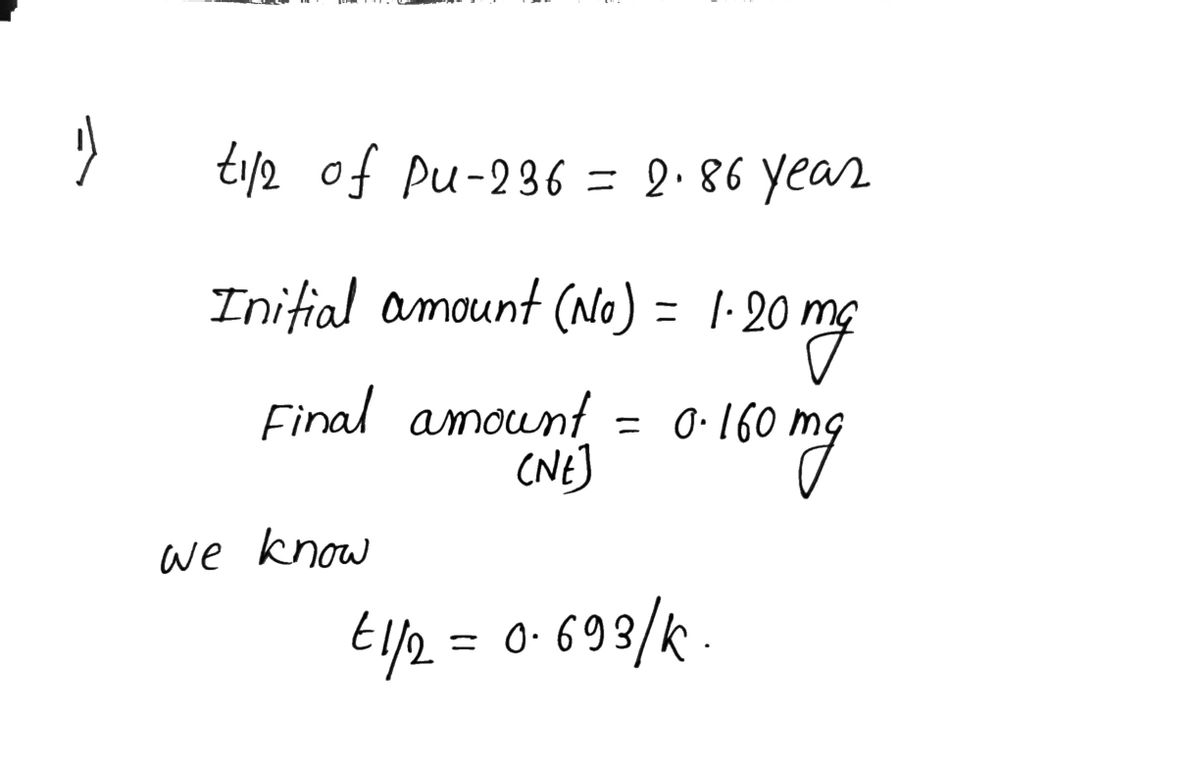
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Radium-223 decays with a half-life of 11.4 days. How long will it take for a 0.240 mol sample of radium to decay 6.00*10^-2 mol?arrow_forwardIodine-131 is administered orally in the form of NaI(aq) as a treatment for thyroid cancer. The half-life of iodine-131 is 8.04 days. If you begin with 40.9 mg of this isotope, what mass remains after 11.7 days have passed?arrow_forwardStrontium-85, used for bone scans, has a half-life of 65 days. How long will it take for the radiation level of strontium-85 to drop to one-fourth of its original level? Express your answer as an integer.arrow_forward
- Iodine-131 is an important radioisotope of iodine that has a radioactive decay half-life and is associated with medical diagnostic and treatment procedures. How many half lives does it take to reach 12.5 % of its original activity?arrow_forwardA moon rock collected by a U.S. Apollo mission is estimated to be 4.00 billion years old by uranium/lead dating. Assuming that the rock did not contain any lead when it was formed, what is the current mass of Pb206 in the rock, if it currently contains 1.430 g of U238? The half-life of U238 is 4.47×109 years.arrow_forwardKrypton-81m is used for lung ventilation studies. Its half-life is 13 ss. How long does it take the activity of this isotope to reach one-quarter of its original value?arrow_forward
- Hydrogen-3 has a half-life of 12.3 years. How many years will it take for 502.0 mg ^3H to decay to 0.49 mg ^3H?arrow_forwardA radioactive sample has a decay constant of 1.32 x 10-3 h-1. What is its half life?arrow_forwardStrontium-89 is a beta emitter with a half-life of 50.6 days that is used in bone cancer treatment. How many days will it take for a 69.6 mg sample of Sr-89 to decay to 0.148 mg? (Assume no excretion of the nuclide from the body).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY