
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
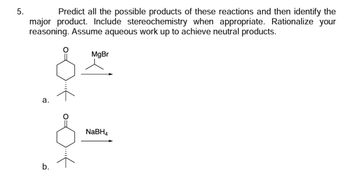
Transcribed Image Text:### Reaction Prediction with Stereochemistry and Aqueous Workup
**Problem 5:**
Predict all the possible products of these reactions and then identify the major product. Include stereochemistry when appropriate. Rationalize your reasoning. Assume aqueous work up to achieve neutral products.
**Reaction A:**
- **Reactant:** A ketone (cyclohexanone derivative) with a methyl group at the beta position.
- **Reagent:** Methylmagnesium bromide (CH₃MgBr).
**Reaction B:**
- **Reactant:** A similar cyclohexanone derivative as in Reaction A.
- **Reagent:** Sodium borohydride (NaBH₄).
### Explanation
1. **Reaction A: Grignard Reaction**
- **Reactivity:** The Grignard reagent (CH₃MgBr) will act as a nucleophile and attack the electrophilic carbonyl carbon of the ketone.
- **Product Formation:** The product will be a tertiary alcohol after aqueous workup, where the magnesium halide is replaced by a hydroxyl group.
- **Stereochemistry:** This reaction is non-stereoselective due to the planar nature of the carbonyl group, leading to racemic mixtures in cases of chiral centers.
2. **Reaction B: NaBH₄ Reduction**
- **Reactivity:** Sodium borohydride will reduce the carbonyl group of the ketone to a secondary alcohol.
- **Product Formation:** The resulting product will be a cyclohexanol derivative.
- **Stereochemistry:** Similarly, reduction of the ketone's planar carbonyl group may lead to racemic mixtures if relevant chiral centers are present.
### Conclusion
- **Major Product Identification:**
- For Reaction A, the major product is the tertiary alcohol after Grignard addition and workup.
- For Reaction B, the major product is the secondary alcohol obtained from the reduction.
Considerations in predicting the products involve the nature of the reagents, type of reaction, and possible stereochemical outcomes.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can you also please provide the minor products of this problems. Thanks!
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can you also please provide the minor products of this problems. Thanks!
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 19. e correct structure for 1-methyl-2,4,.6-trinitrobenzene(TNT) is which of the following? a. NON O2N CH3 CN- -CH3 O2N- NO2 b. d. NO2 CH3 -CH3 ON- -CH3 CH3 GON 20. The correct name for the compound given above is which of the follow ing? a. 2-cycloproproxyleyclobutane b. cyclobutylpropoxy ether с. 2-суcobutохусусlopropanе d. cyclopropoxycyclobutane 21. Of the five names listed below, four contain de liberate errors. Which is the only name is correct? a. 2-pentanal b. 2,3-dichloropentane 3-methy lpropanoic acid d. 1,2-dimethylpropanoate e. n-methy lpropanamide с.arrow_forward2e.arrow_forwardFill in the blanks in the following SN2 reactions with either the appropriate nucleophile,electrophile, or product and include any stereochemistry where it is relevant.arrow_forward
- Predict the products of the following reactions. Include stereochemistry when necessary. For reactions with more than one step show the product formed after each step. OH HO X OH O H PCC 1. NaBH4 2. H* 3. H₂SO4, heat and may not be shared, uploaded or distributed Na₂Cr₂O7 H₂SO4, H₂Oarrow_forwardWhat is the major organic product obtained from the following series of reactions? please draw the reaction. a. heptane b. 1-heptene c.(Z)-2-heptene d. 1,5-heptadienearrow_forwardSuppose you are given the starting molecule shown below. For each of the cases listed below, list the reaction conditions and reagents that would give that product, and give a brief explanation of why ONLY the specified product would be formed under the conditions you chose. Make product alcohol with OH group at carbon 1. Make product alcohol with OH group at carbon 2. Make product alcohol with OH group at carbon 3. а. b. 3 2 C.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY