
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

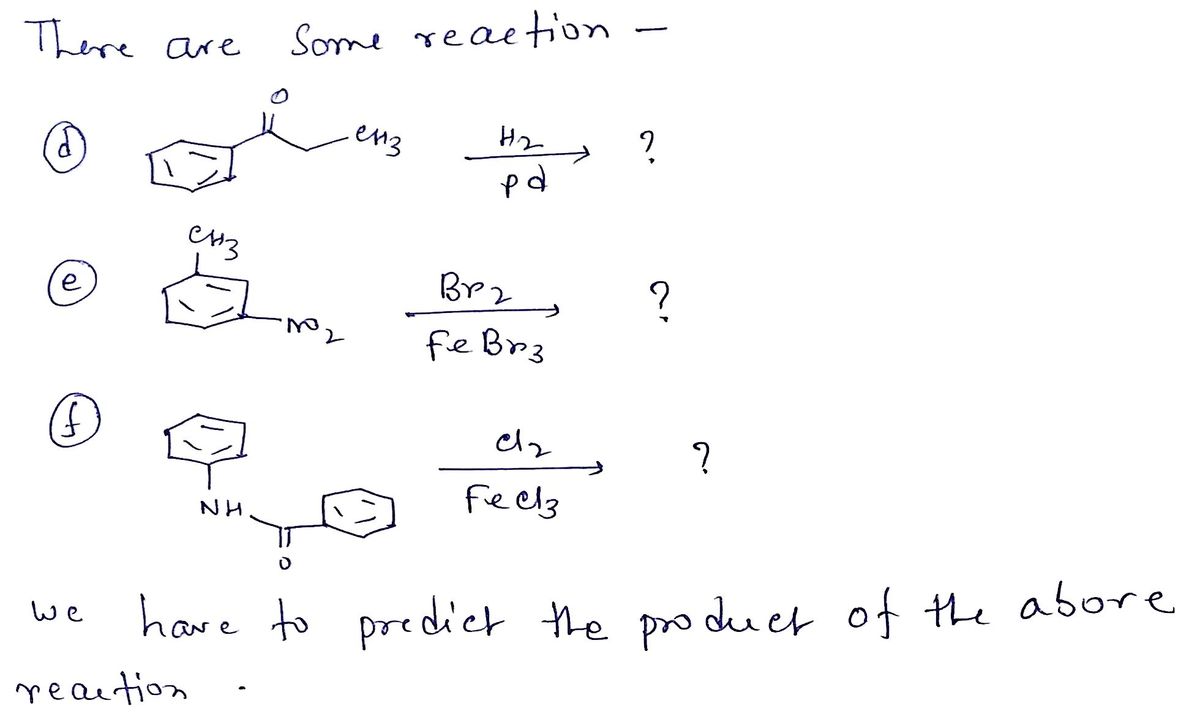
Transcribed Image Text:d)
H,
Pd
e)
Br
Fe,
CH
f)
Fea,
9)
NaOH
h)
NaiH,

Transcribed Image Text:10. Write in the products of these reactions. Include stereochemistry where relevant
Mention ALL products where relevant (ortho/para, etc.)
100-150
CH2
HC
KMNO,
c)
NaOH
340°, 170 atm
CHS
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Aqueous osmium (II) chloride reacts with aqueous cesium carbonate to produce cesium chloride and osmium (II) carbonate. (Osmium has the symbol Os) Solubility Rules Solubility Exceptions Solubility Exceptions insoluble Li", Na", K* , Rb*, Cs*, NH, lon lon nitrates soluble none carbonates sulfates soluble Ca* ,Ba* ,Sr* , Ag* , Pb* insoluble Li, Na', K* , Rb°, Cs*, NH4 phosphates chlorides soluble Ba?", Sr* insoluble Li , Na', K* , Rb°, Cs', NH, *, Ca insoluble Li , Na', K' , Rb°, Cs', NH, ', Mg' Ag°, Pb* hydroxides alkali ions soluble Inone sulfides Ca Ва acetates soluble Inone iodides soluble Ag", Pb²* 1) Write the balance equation including phases (s, I, aq etc.) on the paper with your work that you will submit after the exam. 2) Enter the coefficient in front of the aluminum chloride when the equation is balanced in moodle. The equation you submit must support the number you entered in moodle. Select one: О а. 6 b. 2 с. 5 d. 3 О е.1 O f. 4arrow_forwardF3 4 g # E F4 14 $ R F F5 is 5 F6 T G B The reaction N₂ (g) + 2 O2 (g) → 2 NO2 (g) and therefore heat is 1 A) endothermic, released B) endothermic, absorbed C) exothermic, released F7 JL D) exothermic, absorbed Y H F8 Question 2 of 5 & 7 N Q U DELL F9 * 8 P M I F10 ( 9 K F11 ) O L ΔΗ= 66.4 kJ by the reaction. F12 P PrtScr 1 W @to Insert Delete Backspace Poarrow_forward5. Provide a balanced chemical equation, be sure to include state designations. a. Hydrogen and oxygen react to form steam. b. In an aqueous solution, zinc acetate and sodium sulfide are mixed. c. Lithium and bromine react. d. Combustion of liquid butane (C4H10). e. Dilute sulfuric acid is added to a solution of aluminum hydroxide. f. Decomposition of potassium chlorate.arrow_forward
- In the reaction between FeCl, and NaOH, the spectator ions are A) Fe3 and Ch B) Fe* and OH- C) Na* and OH- D) Na* and CI MacBook Air DII 80 888 F6 FB F9 F10 F2 F3 F4 F5 @ 23 $ & 2 3 4 6 9 W E R Y P S F G H K L C V M - * CO D.arrow_forward2 20 E Solid silver fluoride is slowly added to 150 mL of a potassium chromate solution until the concentration of silver ion is 0.0538 M. The maximum amount of chromate remaining in solution is C Submit Answer $ 4 R FI F V Retry Entire Group 8 more group attempts remaining % Use the References to access important values if needed for this question. 5 T Cengage Learning | Cengage Technical Support G ^ 6 B MacBook Pro Y H & N 20 7 U N ▶II * 00 8 J I M ( 9 K O V. ) O < L ✔ M. P A Previous Email Instructor + Next Save and Exit 21 ?arrow_forwardA. The reaction of elemental potassium and S8 (elemental sulfur exists in its crystalline state as an octadiatomic molecule) to form an ionic compound C. The decomposition reaction of Iron (IV) nitride to form elemental products of solid iron and nitrogen gas.arrow_forward
- A chemical reaction has the equation AgNO3 (s) + NaCI (s) -› AgCI (s) + NaNO3(s). What type of reaction occurs between AgNO3 and NaCl? A. Decomposition B. Synthesis © C. Double displacement D. Single displacementarrow_forwardChemistryarrow_forwardO science 24: Module 1 O bas ( 1 E Assignment Booklet 18 For questions 5 to 9, read each question carefully. Decide which of the choices BEST completes the statement or answers the question. Place your answer in the blank space given. 2 5. Iron oxide (FeO)) seperated into solid iron an example of a 1 (Fe (0)) A. simple composition reaction B. simple decomposition reaction C. combustion reaction D. neutralization reaction 6. In a simple composition reaction, A. one substance combines with another to form a salt and water B. two or more elements combine to form a compound C. a compound is broken down into two or more elements D. water is separated into hydroxgen gas and oxygen gas 7. fuel + oxygen → carbon dioxide + water vapour + energy This word equation is an example of a A. simple composition reaction B. simple decomposition reaction C. combustion reaction D. neutralization reaction A. simple composition reaction B. simple decomposition reaction C. combustion reaction D.…arrow_forward
- See image below....arrow_forwardBalanced chemical equations show________. A. whether a chemical reaction will occur. B. the concentration of reactants and products. C. that molecules are conserved. D. the relative number of reactant molecules which can take part in a chemical reaction.arrow_forwardConsider the given acid ionization constants. Identify the strongest conjugate base. Acid Ka HF(aq) 3.5x10-4 НС-Н: О2 (аq) 6.5х10-5 HC102 (aq) 1.1x10-2 HC2H3O2 (aq) 1.8x10-5 You may want to reference (Page) Section 16.4 while completing this problem.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY