
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
#5
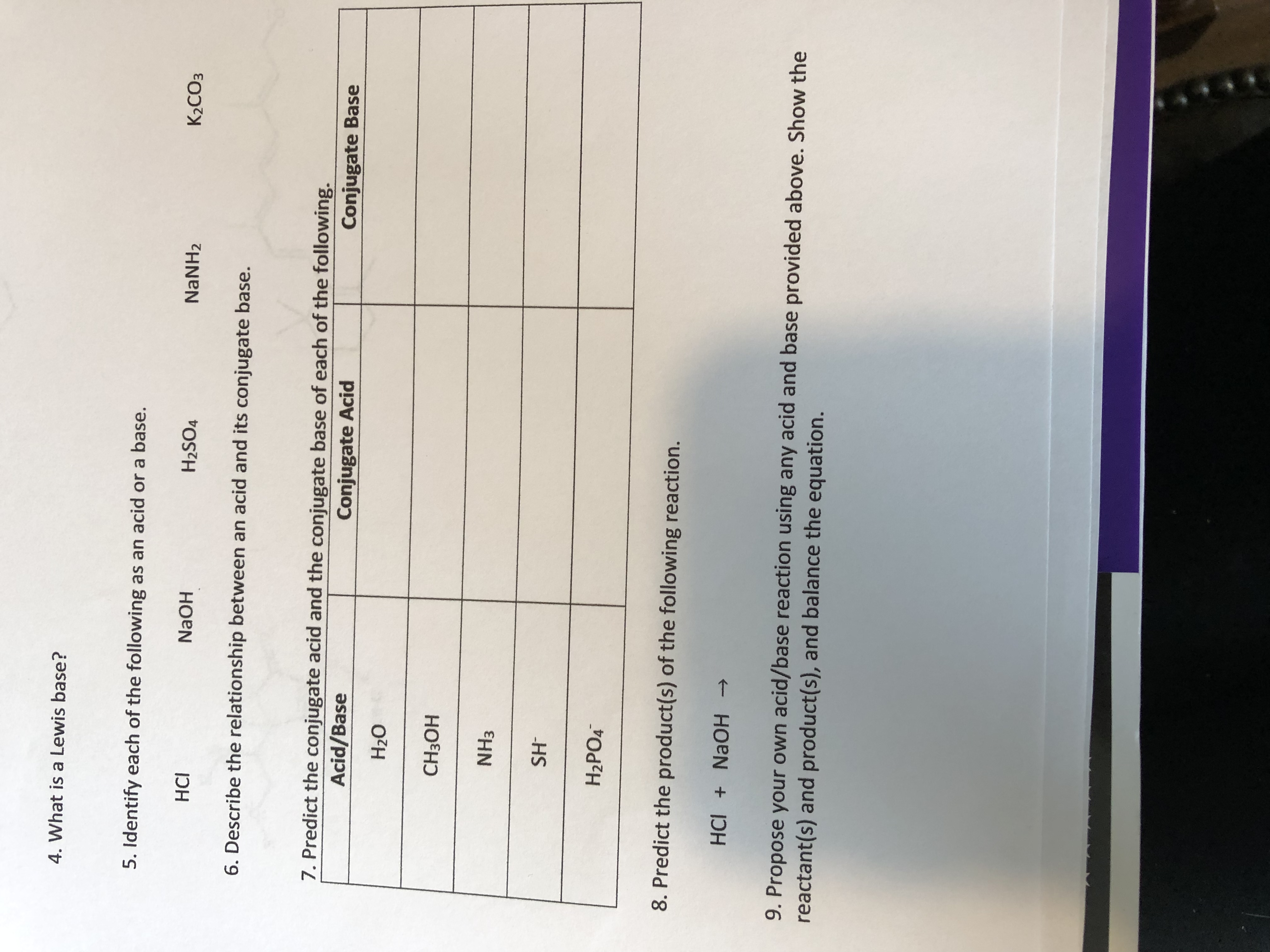
Transcribed Image Text:4. What is a Lewis base?
5. Identify each of the following as an acid or a base.
HCI
H2SO4
NaNH2
K2CO3
6. Describe the relationship between an acid and its conjugate base.
7. Predict the conjugate acid and the conjugate base of each of the following.
Acid/Base
Conjugate Acid
Conjugate Base
HOEH
NH3
HS
H2PO4
8. Predict the product(s) of the following reaction.
HCI + NAOH >
9. Propose your own acid/base reaction using any acid and base provided above. Show the
reactant(s) and product(s), and balance the equation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- # 3 Provide the correct IUPAC name for the compound shown here. 20 F3 $ 4 000 000 F4 de LO % 5 O ůl F5 Question 4 of 29 H-C-CH₂-CH₂-CH₂-C-CH₂-CH3 7,7- 3,3- 7- 3,3- 7- 7- di tert- 6 CH3 CH3 5,5- 5,5- 5- 3- F6 iso tri sec- cyclo oct hex pent eth meth hept MacBook Air & 7 F7 8 DII F8 ( 9 F9 0 F10arrow_forwardQuestion 9 Which of the following is/are secondary (2°) alcohols? ОН HO HO (1) (2) (3) (4) O a) only 1 O b) only 3 O c) only 2 and 4 O d) 1, 2, 3, and 4arrow_forwardH3C F1 2 W F2 3 # E C Consider the molecular compound depicted below. What is its correct (IUPAC) name? DII F3 4x $ 4 R OH F4 4₁ % 5 F5 T D 6 Question 11 of 20 F6 Y F7 & 7 U W PrtScn A) methanone B) methanoic acid C) ethanal D) ethanol E) ethanoic acid F8 8 Home 1 F9 9 End O F10 PgUp F11 aarrow_forward
- Please dear not image written answerarrow_forwardCO 5 FL # 3 AMBRIAN COLLEGE Student Resources Faculty Resources Academic Resources 23 CH3 CH3 What is the IUPAC name for pe CH3-CH-CH-CH2-C-H out of uc Answer: tion 24 What is the IUPAC or common name for this compound? pea CH2-CH3 CH3-CH2-N-CH2-CH3 60 Answer: TIO prt sc home F2 F4 F5 F6 F8 %2$ 2. 6 4.arrow_forward▾ Part A Give the IUPAC name of the following carboxylate salt: 0 CH3–CH,—CH, CH, C-O-K+ Spell out the IUPAC name of the compound. Submit Request Answer ▾ Part B Give the IUPAC name of the following carboxylate salt: CH3 0 CH3-CH,−CH –CH2-C-O-K* Spell out the IUPAC name of the compound. Submit Request Answer Part C Give the common name of the following carboxylate salt: CH3 0 CH3–CH, CH CH,-C-0 K* Spell out the common name of the compound. -arrow_forward
- J C @ 2 F2 W S What is the correct IUPAC name for the compound shown here? CO # 3 F3 e d F4 $ A ܠ 4 f F5 % 5 3- t Question 37 of 49 ISO 9 1- M F6 A 6 5- tert- neo di 2- 6- 4- sec- O DELL y h F7 & 7 F8 j * 8 F9 k F10 0 ) F11 Р F12 Mar 24 JE L + 11 8 deletearrow_forwardHow do I answer part B and part C?arrow_forwardMacmillan Learning # 3 What is the IUPAC name for the compound? H₂C-CH₂-CH-CH₂ E D IUPAC name: 20 $ 4 R LL F Incorrect 2-Methyl butanol-1- Hydroxyl % CH3 5 T Search or type URL G OH ^ 6 tv ♫ MacBook Pro Y H & . ☆ U * 00 8 J + ( 9 K * A 0 ) O L Parrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY