
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
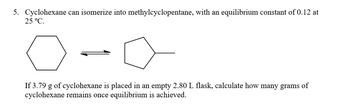
Transcribed Image Text:5. Cyclohexane can isomerize into methylcyclopentane, with an equilibrium constant of 0.12 at
25 °C.
If 3.79 g of cyclohexane is placed in an empty 2.80 L flask, calculate how many grams of
cyclohexane remains once equilibrium is achieved.
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- give handwrittenarrow_forwardO KINETICS AND EQUILIBRIUM Using the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 0.82: co(g) + H,0(g) = co,(g) + H,(g) Use this information to complete the following table. Suppose a 15. L reaction vessel is filled with 0.35 mol of CO, and O There will be very little CO and H,O. 0.35 mol of H2. What can you say about the composition of the mixture in the vessel at equilibrium? O There will be very little CO, and H2. O Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = ] CO,(9)+H,(9) CO(g)+H,O(g) 1L What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = ] 2 CO(g)+2H,O(g) 2 CO,(9)+2H,(9) 1L Explanation Checkarrow_forwardKINETICS AND EQUILIBRIUM Using the general properties of equilibrium constants At a certain temperature, the equilibrium constant X for the following reaction is 0.0099: CO(g) + H₂O(g) = CO₂(g) + H₂(g) Use this information to complete the following table. Suppose a 16. L reaction vessel is filled with 0.60 mol of CO₂ and 0.60 mol of H₂. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. CO₂(g) + H₂(g) → CO(g) +H₂O(g) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 CO(g) +3H₂O(g) 3 CO₂(g)+ 3H₂(9) There will be very little CO and H₂O. There will be very little CO₂ and H₂. Neither of the above is true. K = 0 K = 0 □ x10 X 3/5arrow_forward
- The equilibrium constant, Kc, for the following reaction is 83.3 at 500 K.PCl3(g) + Cl2(g) PCl5(g)Calculate the equilibrium concentrations of reactant and products when 0.271 moles of PCl3 and 0.271 moles of Cl2 are introduced into a 1.00 L vessel at 500 K.arrow_forward= KINETICS AND EQUILIBRIUM Setting up a reaction table Suppose a 500. mL flask is filled with 1.7 mol of N₂ and 0.10 mol of NH3. This reaction becomes possible: N₂(g) + 3H₂(g)2NH, (g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of N₂. You can leave out the M symbol for molarity. initial change equilibrium N₂ 0 0 H₂ 0 0 0 NH3 0 0 00 1/5 Xarrow_forwardConsider the following equilibrium system: 2SO2 + O2 --> 2SO3 Into a 2.00 Liter container, 0.500 mol of SO3, was placed and heated to 300°C. After equilibrium was established, the container was found to have 0.300 moles of SO3 present along with the other gases. Calculate the equilibrium concentration of each molecule and the equilibrium constant for this reaction at this temperature.arrow_forward
- The equilibrium constant, Kc, for the following reaction is 57.6 at 277 K. 2CH₂Cl₂ (g) CH4 (9) + CCl4 (9) When a sufficiently large sample of CH₂Cl₂ (g) is introduced into an evacuated vessel at 277 K, the equilibrium concentration of CC14 (g) is found to be 0.383 M. Calculate the concentration of CH₂Cl₂ in the equilibrium mixture. [CH₂Cl₂] = Marrow_forward2. 1.00 mol of oxygen and 1.00 mol of sulfur dioxide are placed into a 1.0-L flask. Sulfur trioxide gas is formed. At 1000 K, when equilibrium has been reached, 0.93 mgl pf sulfur trioxide have been formed. What is the Kec?.arrow_forward#3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY