
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
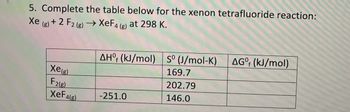
Transcribed Image Text:5. Complete the table below for the xenon tetrafluoride reaction:
Xe (g) + 2 F2 (g) → XeF4 (g) at 298 K.
Xe
AHO (kJ/mol) S° (J/mol-K) AGº (kJ/mol)
169.7
202.79
XeF4(g)
-251.0
146.0
F2(g)
f
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Consider the following thermochemical equations. Reaction ΔrH° / kJ mol−1 HBr(g) ⟶ H(g) + Br(g) 365.7 H2(g) ⟶ 2 H(g) 436.0 Br2(g) ⟶ 2 Br(g) 193.9 What is ΔrH° for the reaction below? HBr(g) ⟶ ½ H2(g) + ½Br2(g) Give your answer in kJ mol−1, accurate to one decimal place. Kindly double check your solution.arrow_forwardBelow is the reaction for the evaporation of water. H2O (I) H20 (g) AH°f H2O (1)= -285.8 kJ/mole ; H20 (g) = -241.8 kJ/mole %3D Calculate AH*rxn for this process. This is the reaction that happens when your sweat evaporates. Why is this a cooling mechanism for your body? How much sweat would have to evaporate from a person's skin to cool the body by 0.5 C? Assume this person has a body mass of 150 Ibs and the specific heat capacity of the body is 4.0 J/g °C. 1 Ib. = 0.454 kgarrow_forwardIdentify the reactions that are exothermic. ) Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation... SHOW MORE V C(graphite)C(diamond) AH° = 2 kJ b. CaCO3 (s) CaO(s) + CO2(g) AH° = 178 kJ CuO(s) + H2 (g) → Cu(s) + H2 0(g) AH° = -85 kJ 2H2 (g) + 02(g) - 2H20(g) AH = -484 kJ 3H2 (g) + N2(g) 2NH3 (g) AH - 92 kJ Unansweredarrow_forward
- Determine the AU°reaction at 25 °C (in kJ/mol) for the following reaction: 2NO(g) + O2(g)→2NO2(g) Appendix D kJ/molarrow_forwardConsider the reaction: 2 H,0(g) → 2H,(g) + 0,(g) If 2.0 moles of H2O(g) are converted to H2(g) and 02(g) against a pressure of 1.0 atm at 125 °C, what is the change in internal energy for this reaction? R-8.314 1/mol K AH- 483.6 kl/molarrow_forwardFor a particular reaction at 180.9 °C, AG = 527.78 kJ/mol, and AS = 953.96 J/(mol · K). Calculate AG for this reaction at -3.7 °C. AG = kJ/molarrow_forward
- Consider the reaction. 2Fe2O3⟶4Fe+3O2Δ?∘rxn=+824.2 kJ2Fe2O3⟶4Fe+3O2ΔHrxn°=+824.2 kJ The formation of 75.0 g75.0 g of O2 results in the absorption of 1930 kJ1930 kJ of heat. the absorption of 20600 kJ20600 kJ of heat. the release of 20600 kJ20600 kJ of heat. the release of 644 kJ644 kJ of heat. the release of 1930 kJ1930 kJ of heat. the absorption of 644 kJ644 kJ of heat.arrow_forwardCalculate AS°rxn for the following reaction. The S° for each species is shown below the reaction. N2H4(l) + H2(g) → 2 NH3(g) ings S°(J/(mol·K)) 121.2 130.7 192.8 O -59.1 J/K es O 311.0 J/K O 133.7 J/K O -133.7 J/K O 637.5 J/Karrow_forwardwhat letter choice is thisarrow_forward
- What is AS for the following reaction? 2Cl2(g) + SO2(g) SOCI₂(g) + Cl₂O(g) Substance: Cl₂(g) SO2(g) SOCI2(g) C1₂O(g) 223.0 248.1 309.8 266.1 S(J/K. mol):arrow_forwardThe value of AH° for the reaction below is -336 kJ. Calculate the heat (kJ) released to the surroundings when 23.0 g of HCl is formed. 26. CH4 (g) + 3Cl, (g) → CHC1; (1) + 3HC1 (g) A) B) C) 211 177 70.7 D) -336arrow_forward9.An _____ reaction results when the energy released by the formation of products is greater than the energy required to break the bonds in the reactants. Group of answer choices A, endothermic B, exothermic C, exergonic 10,The following is correct for the chemical reaction taking place at constant volume: Group of answer choices A, ΔE = 0 B, ΔH = ΔE + P ΔV C, ΔE = q D, ΔE = ΔHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY