
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
The question is included on this one, please help me to understand and solve for these two problems. My answers are not matching what it should be! (On both of these multiple choice questions) Thank you!
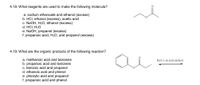
Transcribed Image Text:4.18. What reagents are used to make the following molecule?
a. sodium ethanoate and ethanol (excess)
b. HCI, ethanol (excess), acetic acid
c. NaOH, H2O, ethanol (excess)
d. HCI, H2O
e. NaOH, propanol (excess)
f. propanoic acid, H20, and propanol (excess)
4.19. What are the organic products of the following reaction?
a. methanoic acid and benzene
b. propanoic acid and benzene
c. benzoic acid and propanol
d. ethanoic acid and phenol
e. phenylic acid and propanol
f. propanoic acid and phenol
H2O (+ an acid catalyst)
Expert Solution
arrow_forward
Step 1
The 1st reaction is an esterification reaction.
In this reaction, alcohol and carboxylic acid reacts on the addition of a small amount of acid to give respective esters which are sweet-smelling compounds.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- doug began preparing laboratory surface disinfectant from chlorine bleach. he put on a chemical resistant apron and gloves and then removed the bleach container from the special chemical cabinet. he carefully placed the container on the laboratory benchtop and began to add the chlorine bleach to distilled water. nearby workers began complaining of burning eyes. doug was reprimanded by the supervisor. Explain why.arrow_forwardWhy are ducks waterproof? It’s because they produce copious amounts of oils from their uropygial glands and spread it across their feathers. In this exercise, we’ll be investigating the molecular structure of one of these preen oils to determine how it keeps ducks dry. Q.5 - Preen oil is actually a complicated mixture of many different organic compounds, such as the structure seen previously.. Ornithologists have determined that birds often use preen oil compounds for scent recognition. Below, several different chemicals isolated from preen oil are shown, along with their vapor pressures at room temperature. p-cymene has the highest vapor pressure, meaning it is the most easily evaporated compound of the three listed. Explain why p-cymene has a higher vapor pressure at room temperature compared to the other compounds. Make sure to explain what holds the p-cymene in the sample. (Image attached)arrow_forwardIs the following statement true or false? If you think the statement is false, rewrite it to make it true: Closed loop manufacturing involves the recycling of wastes so they are not released back into the environment. NOTE: This question is related to green chemistry in the industry.arrow_forward
- rch ||| O CHEMICAL REACTIONS Predicting the products of a neutralization reaction Predict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced. HNO3 + NaOH → 0 Explanation Ease RK- burial WE burial Check papers 46 H. Kdy go ahead X 09 1/5 Christopher Mitchell Jessica V 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 000 14 GEarrow_forwardThe above solution was wrong when entered into homework sitearrow_forwardEach sketch below shows three objects with an electric charge. In each case, decide whether there is a net force acting on the object outlined in green. If there is a net force, decide whether it pushes the green-ring object to the left or right. Then select the appropriate button under the sketch. For example, if there is a net force pushing the green-ring object in the first sketch to the left, select the left button under the first sketch. If there is no net force on the green-ring object in the second sketch, select the middle button under the second sketch. And so on. ola -2 +3 -2 -2 I Don't Know Submit 2022 McGraw Hill LLC. AL Riahts Reserved. Terms of Use I Privacy Center Accessibility etv 16 Dl 18 80 & % 6 7 8O 4.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY