
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Help?
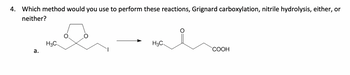
Transcribed Image Text:4. Which method would you use to perform these reactions, Grignard carboxylation, nitrile hydrolysis, either, or
neither?
H3C
a.
H3C
COOH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps

Knowledge Booster
Similar questions
- A doctor has ordered for a week 0.35 grams of Carvedilol for a patient with high blood pressure. If your stock on hand consists of 25 mg tablets, how many tablets will you need for 1 day's treatment for the patient? QUESTION 13 O a. 2 O b. 14 O C. 7 O d. 20 O e. 1 QUESTION 14arrow_forwardHow does magnesium hydroxide provide relief for indigestion? Pick from the following • Magnesium hydroxide is an acid that digests food in the stomach. • Magnesium hydroxide produces magnesium chloride salt, which neutralizes HCI. • Magnesium hydroxide reacts with HCI to produce a solution that is neutral. • Magnesium hydroxide is an acid that neutralizes HCI in the stomach.arrow_forwardFor sodium bicarbonate (baking) explain the hazards effects of being exposed to this chemical either by contacting the skin, swallowing it, or contacting your eye. What should you do then as first aid and, describe how you should dispose off those chemicals.arrow_forward
- Part IV. Henry's Law More Carbon dioxide gas will dissolve (or stay dissolved) in water when pressure is (high or low?) .Dissolved Carbon dioxide leaves water when the pressure is (high or low?)arrow_forwardPls help ASAParrow_forwardA 1:20 dilution is done with a 0.2500 M Na,SO, solution. What is the concentration of the diluted solution ? A. 0.5000 M B. 0.0500 M C. 0.1250 M D. 0.01250 Marrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY