
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Answer for number 4
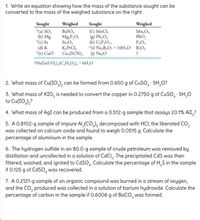
Transcribed Image Text:1. Write an equation showing how the mass of the substance sought can be
converted to the mass of the weighed substance on the right.
Sought
"(a) SO,
(b) Mg
*(c) In
(d) K
"(e) Cuo
Sought
(f) MnCl,
) Pb,O,
(h) U,P,O1
*6) Na,B,O, · 10H,O B,O,
Weighed
Weighed
BaSO,
Mg,P,O,
In,O,
K,PtCl,
Cu,(SCN); () Na,O
Mn,O,
PbO,
P,O,
1NAZN(UO,),(C,H,0), · 6H,0
2. What mass of Cu(IO,), can be formed from 0.650 g of CUSO, · 5H,0?
3. What mass of KIO, is needed to convert the copper in 0.2750 g of CUSO, · 5H,0
to Cu(IO,),?
4. What mass of AgI can be produced from a 0.512-g sample that assays 20.1% AlI,?
5. A 0.8102-g sample of impure AI,(CO,), decomposed with HCI; the liberated CO,
was collected on calcium oxide and found to weigh 0.0515 g. Calculate the
percentage of aluminum in the sample.
6. The hydrogen sulfide in an 80.0-g sample of crude petroleum was removed by
distillation and uncollected in a solution of CdCl,. The precipitated CdS was then
filtered, washed, and ignited to CdSO. Calculate the percentage of H,S in the sample
if 0.125 g of CdSO, was recovered.
7. A 0.2121-g sample of an organic compound was burned in a stream of oxygen,
and the CO, produced was collected ina solution of barium hydroxide. Calculate the
percentage of carbon in the sample if 0.6006 g of BaCO, was formed.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY