
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I need help with questions 4-6?

Transcribed Image Text:1. Find the overall reaction that will be occurring between the H₂B03/ B and
Bio+ / Bi half cells in acidic conditions.
2. What is the standard cell voltage of this electrochemical cell?
3. What is the standard free energy of this reaction?
4. What is the equilibrium constant of this reaction at 25 °C?
5. After 56.0 hours at 1.04 A, how many moles of electrons have been used in this
reaction?
6. Predict the sign of the entropy (ASrxn) for this electrochemical reaction. Explain your
reasoning.
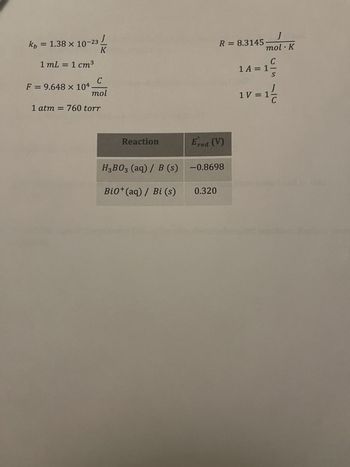
Transcribed Image Text:x
kb = 1.38 × 10-23/
K
1 mL = 1 cm³
C
mol
1 atm = 760 torr
F = 9.648 x 104
Reaction
H₂B03 (aq) / B (s)
Bi0+ (aq) / Bi (s)
R = 8.3145
Ered (V)
-0.8698
0.320
J
mol. K
A
14=1²
1V=1/2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Kw=1.0x10-¹4 Report all pH values to two places past the decimal. 1. Write the following acid/base reactions. Circle the bases and underline the acids. a. hypochlorous acid (HCIO) reacts with water b. the base ammonia (NH3) reacts with waterarrow_forward7 Complete the table below. Be sure each of your answer entries has the correct number of significant digits. You may assume the temperature is 25 °C. 4 W formula NH 80°F Partly sunny 0 conjugate acid Continue HCN 3 E Ka 5.6 x 10 0 X 4.9 × 10 -10 $ 4 R -10 с formula F5 HS % 5 T 0 conjugate base H Q Search F6 6 K₂ 0 1.8 x 10 F7 87 & F8 Do X J * 8 - F9 a x10 F10 ( 9 Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cen C O - F11 ) 0 *+ F12 PrtSc + 11 Insert Subr = Delete Backsparrow_forwardI need help with this questionarrow_forward
- Please answer number 4-8arrow_forward1 Chemistry Time's Up! What is the number of moles of glucose (C₂H₂O) in 0.500 L solution? ADD FACTOR 1-210 Sections HA LECTU X 0.20 0.500 mL ANSWER 0.020 36.04 1.3 M glucose g glucose mol glucose MacBook Air 1 180.18 of a 0.40 6.022 x 1023 L M RESET PUL C 0.40arrow_forwardSTARTING AMOUNT esc C X C 2 F2 W # 3 1000 F3 ADD FACTOR x( ) $ 4 1 97.9 FS de in % 5 2 Convert 23.4 kJ to calories 2.34 x 104 t 4184 kcal 6 P 0.001 J 4.184 COMEC DOLL ANSWER & 7 9.79 × 104 cal 00 8 RESET FO 2 23.4 ( 5590 A FIT 120 Jan 21 del +arrow_forward
- 1. (answer the questions in the tan boxes in the tables below each question) A student conducted an investigation to determine the effect of water temperature on the amount of sugar that dissolves in a beaker of water. Identify components for trial 1 of this investigation. Beaker Number 1 2 3 4 Amount of Water (mL) 100 100 100 100 Amount of Water (mL) Trial 1 Temperature of Temperature of Amount of Sugar Sugar (°C) Water (°C) Dissolved (g) 20 20 20 20 Temperature of Sugar 5 10 15 20 185 189 194 204 Temperature of Water Terms Variable Constant Amount of Sugar Dissolvedarrow_forwardConcentration (M) 1 0.8 0.6 0.4 0.2 0 0 1st attempt 10 y = -0.0111x + 1.0362 20 30 Time (minutes) What is the y-intercept of this graph? 40 50 60arrow_forwardHP TrueVision HD 4 Texas Workfor Mộc Homework × W. College Physic mical App AN Careers eagleonline.hccs.edu/courses/188795/assignments/3601808 valnabie diler reD 4 dl 10.1Tam Constants | Periodic Table Part O Complete the fourth row. Complete the following table by calculating the missing entries and indicating whether the solution is acidic or basic. Express your answer using two decimal places. Acidic or [H+] [OH-] pH pOH basic? 7.4x10-3 ΑΣφ 3.3x10-10 M pH 8.25 5.73 Is to fa ho II 10 insertarrow_forward
- 4-16. Zinc is an essential micronutrient in pet food, but is toxic if present in excess. Do the Zn concentrations (mg/g) for the five cat foods and two dog foods determined by two methods differ significantly at the 95% confidence level? Cat 1 Cat 2 Cat 3 Cat 4 Cat 5 Dog 1 Dog 2 Old method: bris 8 Я 84.9 73.5 173.0 62.7 154.0 80.1 185.0 New faster method: 86.2 81.8 186.0 73.4 138.0 72.5 203.0 SO Souza, S. S. L. Costa, R. G. O. Araujo, C. A. Cats andarrow_forward2- 185.0 mL of .12 M C2H5NH2 with 285.0 mL of 0.21 M C2H5NH3CI Kb(C2H5NH2) = 5.6×10^-4 Express your answer using two decimal places. pH = ?arrow_forwardPlease answer questions 3 and 4 and show work please. Thank youarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY