
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
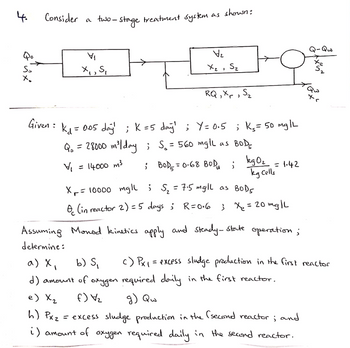
Transcribed Image Text:4.
Qo
S₂₂
Consider a two-stage treatment system as shown:
VI
X₁, S₁
( )
Given :
V₂
X₂ S₂
1
RQ, Xr, S₂
k₁= 0.05 day; K=5 day¹; Y=0.5 ; K₂ = 50 mg/L
Q₁ = 28000 m³/day ; S₂ = 560 mglL as BOD
= 14000 m³ ; BOD ₁ = 0.68 BOD₁;
V₁
kg 0₂ = 1.42
kg Cells
X₁ = 10000 mg/l ; S₂ = 7.5 mgll as BOD5
(in reactor 2) = 5 days; R=0.6
; x₂ = 20 mg/L
Assuming Monod kinetics apply and steady-state operation;
determine:
uxy
h) Px z
= excess sludge production in the (second reactor; and
i) amount of oxygen required daily in the second reactor.
Qw
+く
Qu
a) x₁
b) S₁
c) Px₁ = excess sludge production in the first reactor
d) amount of oxygen required daily in the first reactor.
f) V/₂
g) Qw
e) X₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Similar questions
- determine the minimum H equation and the optimum μ by differentiating the van deemter equation by calculating dH/dμarrow_forward2.4 The sampling train indicates that the concentration of SO2 in a stack is 400 ppm. The pitot tube and manometer in the same train indicates that the flow velocity is 14 m/s. The stack diameter is 2 m. the stack gas temperature and pressure are 505 K and 1 atm. What is the SO2 flow rate?arrow_forwardCalculate the velocity of fluidization of a catalytic bed of diameter D = 2ft and initial height Ho= 3ft. The bed is packed with solid particles of density p, = 2 g/cm³and effective diameter Dp = 0.5cm, under continuous flow of fluid of viscosity µ = 1cP and density på = 1 g/cm³. The void fraction, ɛ, equals ɛ = 1- n/6 (cubic arrangement of particles). What power input is required at fluidization?arrow_forward
- Darrow_forwardREDMI NOTE 6 PRO MI DUAL CAMERA + て(ん- NニES5 )+2(355.94-325.5) k5/m 355mls· %3D ーュ= AV GAYI A=ー 65ラ、 %3D 2: At steady state, air at 200 kPa, 52 C and a mass flow rate of 0.5 kg/s enters an insulated duct having differing inlet and exit cross- sectional areas. At the duct exit, the pressure of the air is 100 kPa, the velocity 225 m/s and the cross sectional area is 2x10 m. Assuming the ideal gas model, determine (a) The temperature of the air at the exit in C. (b) The velocity of the air at the inlet in m/s. 2. (c)The inlet cross-sectional area in m'arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The