
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
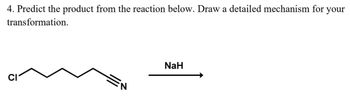
Transcribed Image Text:4. Predict the product from the reaction below. Draw a detailed mechanism for your
transformation.
NaH
CI
N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- g) h) i) j) Br Br OCH₂ DMSO HOCH, H₂O NaOCH DMSOarrow_forwardSynthesis Using reactions we have studied up to this point, propose a multistep synthesis to make the product from the starting material. You will need 3-4 steps.arrow_forwardThe transformation below takes place by two distinct reactions. Intermediate A is formed in the first reaction and then this goes on to the product in the second reaction. Provide a complete curved-arrow mechanism for all steps of both reactions.arrow_forward
- show me and explain the mechanism for this reaction pleasearrow_forwardThe following transformation involves a conjugate addition to an unusual alkenyl phophonium ion. Provide a detailed mechanism for this transformation, including all proton transfers, intermediates, and byproducts.arrow_forwardPlease show mechanismarrow_forward
- OH CH3OH HⓇ OCH 3 In the space provided below please show a detailed, stepwise mechanism for the transformation shown above. You must show all intermediates (including proton transferarrow_forwardComplete the following reaction by mechanism E1 and E2. Present all steps and all possible products in each mechanism. See the image.arrow_forward2- Mention the product of a nucleophilic substitution reaction of (S)-2-bromohexane with acetate ion, CH3CO2- Assume that inversion of configuration occurs, and show the chemistry of both the reactant and product. 3- Draw the mechanism of the SN2 Reaction. Show the correct directions of the arrows, and all reagents. 4- Draw the mechanism of the SN1 Reaction. Show the correct directions of the arrows, and all reagents. 5- Draw the mechanism of the E2 Reaction with an Alkyl Halide. Show the correct directions of the arrows, and all reagents. 6- Draw the mechanism of the E1 Reaction with an Alkyl Halide. Show the correct directions of the arrows, and all reagents. 7- Mention the product formed in an SN2 reaction between 1-bromobutane and NaI. 8- Rank the following compounds in order of their expected reactivity toward SN2 reaction: CH3Br, CH3OTos, (CH3)2CHCl. 9- Explain Grignard Reagents in details with one example. 10- Mention how a halogen substituent can be replaced by a deuterium atom…arrow_forward
- Propose a mechanism for this transformation. Add any remaining curved arrow(s) to complete step 1 of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in this step. H. P HÖ: HO он + 1-ÖH; F CI + Br + ----arrow_forwardHello, predict the product and show the mechanism for each reaction below. What do the mechanism have in common and why?arrow_forwardNitesharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY