
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
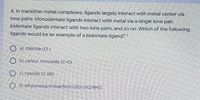
Transcribed Image Text:4. In transition metal complexes, ligands largely interact with metal center via
lone pairs: Monodentate ligands interact with metal via a single lone pair,
bidentate ligands interact with two lone pairs, and so on. Which of the following
ligands would be an example of a bidentate ligand?
O a) chloride (CH-)
O b) carbon monoxide (C=0)
c) cyanide (C EN)
O d) ethylenediammine(NH2-CH2-CH2-NH2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which one of the following is true regarding ligands? An example of a monodentate ligand is NH3 None of these are true statements Ligands are either positively charged or contain an empty electron orbital A chelator can be any type of ligandarrow_forwardDimethyldithiocarbamate (abbreviated as dmdtc) is an anionic bidentate ligand with a chemical formula (S2CNMe2)- - where Me stands for methyl group (-CH3). In the photo above, this ligand coordinates to a metal through its 2 sulfur atoms (where M represents a metal). When three dmdtc ligands bind to a ferric ion (Fe3+), a stable coordination complex called ferbam is formed. Given this information, answer / identify the following. complex geometry hybridization type of the central atom coordination numberarrow_forwardWhich of the following is a necessary characteristic of a ligand? (there may be more than one correct answer) A ligand must be an amine. A ligand must be high spin. A ligand must be a Lewis base. A ligand must be an electron pair donor. A ligand must be an anion.arrow_forward
- 26. Which of the following statements about EDTA (shown below) are FALSE? 28060 HG It can act as a chelating ligand. It is often used to treat heavy metal poisoning. It coordinates to metals through the N and O atoms. It cannot act as a bridging ligand. It is a hexadentate ligand.arrow_forwardDenticity Denticity is the number of atoms in a ligand that bind to a central atom in a coordination complex. H H For example, ethylene diamine (shown on the right) has two nitrogen atoms with lone pairs that can both bind to a metal. This ligand is bidentate. N: Identify the number of atoms in each ligand that can donate a lone pair. N: O monodentate O not a ligand O bidentate O bidentate O not a ligand O monodentate CH3 O bidentate O not a ligand O monodentate OH O bidentate O monodentate O not a ligandarrow_forwardPlease answer my question!!arrow_forward
- 4)arrow_forwardThe color for each “hexaaqua” complex is given below. Select the ligands thatcould change the color of the complex to the color in column 2.Hint: The spectrochemical Series will come in handy here.I− < Br− < Cl− < F− < OH− < H2O < SCN− < NH3 < en < NO2− < CN− < COarrow_forwardFor each of the following compounds identify the ionization sphere the coordination spere the central metal ion the ligands the primary valence the secondary valence a). [Cr(H2O)2 I2] NO3 b). Ca2[Fe(NO2)6] c). [Ni(NH3)3 (CN)2] d). [Au(H2O)2] IO3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY