
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
question is attached. thank you
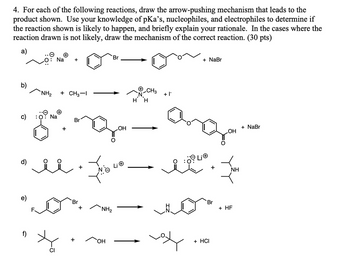
Transcribed Image Text:4. For each of the following reactions, draw the arrow-pushing mechanism that leads to the
product shown. Use your knowledge of pKa's, nucleophiles, and electrophiles to determine if
the reaction shown is likely to happen, and briefly explain your rationale. In the cases where the
reaction drawn is not likely, draw the mechanism of the correct reaction. (30 pts)
a)
+
Na +
Br
NH2
+ CH3-1
+ |¯
H H
Na
+
Br
OH
+
Br
+
NH₂
+
OH
CI
+ NaBr
+
+ NaBr
OH
Br
+ HF
+ HCI
NH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Can you please show the product of these three reactions and show the COMPLETE mechanisms that reflect the electrophiles, nucleophiles, proton transfers, and nucleophilic attacks? Be sure to include proper charges where indicated. (3) OH FO OH OH H НО. H₂SO4 acetone H₂SO4 T methanol H₂SO4 Parrow_forwardIn the experiment where an Electrophillic aromatic substitution sulfonation reaction is conducted on Indigo to produce the food dye FD&C Blue #2(indigo blue or indigotine). show a mechanism showing a single sulfonation on a molecule of indigo.arrow_forwardA chemist in need of 2,2-dimethylpentanoic acid decided to synthesize some by reaction of 2-chloro-2-methylpentane with NaCN, followed by hydrolysis of the product. After the reaction sequence was carried out, however, none of the desired product could be found. What do you suppose went wrong?arrow_forward
- Yead. III. Show a complete synthesis of the following molecule starting with cyclohexane (as many times as you wish), any molecules of three (3) carbons or fewer, and any other reagents. (16 pts) Zoxo 5 Br BV-Br +Br HBV Br-Br Br Br Br Br Br HBV Br Br Brz ← ·Br Br BL Δ H G HO) TH F -OH он してarrow_forwardq, a Proton transfer d = Electrophilic addition g = S 1 Nucleophilic substitution b = N 'N' Lewis acid/base e = E1 Elimination h = S_2 Nucleophilic substitution c = Radical chain substitution f = E2 Elimination Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 12arrow_forwardPlease give a reasonable mechanism for the following reaction using a multiple component reactions. Please draw it out and write out the steps. PLEASE SHOW WITH ARROWS. 5 PhNH + n-Bu 10 mol% TI (NM)2(doma) + ABU-NC tol 100 °C, 48 h, 77% Bu n-Buarrow_forward
- h- Syl Nucleophilic substitution i- Sy2 Nucleophilic substitution j- Electrophilic aromatic substitution a - Proton transfer e - Electrophilic addition b- Lewis acid/base f-E1 Elimination c- Radical chain substitution d = Radical chain addition. g- E2 Elimination Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - j for your answers. HI AICI3 Cl- AICI, OEt Na OEt OEt HOET Na AIBN CN 70° poly(acrylonitrile) (Orlon, Acrilan)arrow_forwardUse the information below to identify and explain the relationship between the basicity of the two nucleophiles and their nucleophilicitiesarrow_forwardWhich of the following statements is TRUE regarding the reaction below? Options: The IR spectrum of the major organic product will show a broad absorption between 3000-3500 cm-1. The mass spectrum of the major organic product will show an M+2 peak in the molecular ion region. The reaction should proceed without carbocation rearrangement. The alkene is the electrophile and water is the nucleophile in the first step. HSO4- is the dominant nucleophile in the second step.arrow_forward
- How to attempt this problem?arrow_forwardUse the reaction given below to answer the following questions. A + р CH3OH 12pt Paragraph B H₂O+ C a) What are the approximate pKas of compounds A and B? b) The reaction given above is irreversible. Give a detailed explanation of the reaction and reaction mechanism. Your explanation should indicate your knowledge of the reactivity of carboxylic acid derivatives. BIU + CH3NH3+ D V 0 wordsarrow_forwardHow to attempt this problem?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
