
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
In the following blanks, write the chemical formula of the named coordination compounds. Use the proper case for the symbol of each atom/ligand and normal numbers, no subscripts or superscripts. Observe proper spacing and use of parenthesis and brackets.
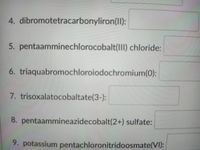
Transcribed Image Text:4. dibromotetracarbonyliron(II):
5. pentaamminechlorocobalt(1II) chloride:
6. triaquabromochloroiodochromium(0):
7. trisoxalatocobaltate(3-):
8. pentaammineazidecobalt(2+) sulfate:
9. potassium pentachloronitridoosmate(VI):
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the following blanks, write the chemical formula of the named coordination compounds. Use the proper case for the symbol of each atom/ligand and normal numbers, no subscripts or superscripts. Observe proper spacing and use of parenthesis and brackets.arrow_forward(b) Explain the bonding in coordination compounds in terms of Werner's postulates.arrow_forwardDear Hand written not allowed.arrow_forward
- Chemistry Combinations of cobalt (III), ammonia, nitrite ions, and potassium ions result in the formation of a series of seven coordination compounds with the coordination number of cobalt equal to six and the ligands bonding to the central atom through N. Give the formulas and names of ALL the seven compounds in the series.arrow_forwardHexaamminechromium(III) ion, [Cr(NH³)]³+ is a metal complex containing six ammonia ligands i. ii. Using the Valence Bond Theory, construct the hybridization state of [Cr(NH3)6]³+. Predict the observed colour of the complex ion if the absorbed colour is blue-violet and briefly explain your answer.arrow_forwardWrite the types of isomerism in coordination chemistry.arrow_forward
- 4. For each of the following transition metal complex ions (a) [FeBr2(OH)(NO3)]¹- (b) [RhF3(SCN)3]4- (c) [TiBr4]³- (i) Draw the structure of the transition metal complex ion. Show the charge on the complex in your structure. Give the oxidation state of the metal. State whether the complex is high spin or low spin or not applicable. Provide reasons for your answer. (iv) Draw the arrangement of d-electrons in the ground state. Label the orbital sets. (v) Calculate the LFSE (in units of Ao or AT) for each complex. (ii) (iii)arrow_forwardWhich statements are true regarding ligands? Select all that apply. Ligands donate all electrons involved in bonds they form with metal cations. Ligands are electron pair acceptors in coordination complexes. Ligands act as Lewis bases in coordination complexes. Ligands form ionic bonds with transition metals. All ligands with two lone pairs are bidentate. A ligand that can donate more than one electron pair to a metal cation is called a chelate. One ligand can donate only one lone pair to a metal cation.arrow_forwardGive the chemical formula of the following coordination compound. Enclose complexes in square brackets, even if there are no counter ions. Do not enclose a ligand in parentheses if it appears only once. Enter water as H₂O. Use any abbreviation given in blue below the question, and enclose it in parentheses if there is more than one. Compound Name tetraaquadihydroxonickel(II) Formulaarrow_forward
- Give the chemical formula of the following coordination compound. Enclose complexes in square brackets, even if there are no counter ions. Do not enclose a ligand in parentheses if it appears only once. Enter water as H₂O. Use any abbreviation given in blue below the question, and enclose it in parentheses if there is more than one. Compound Name tris(ethylenediamine)zinc(II) iodide H₂N CH₂-CH₂ NH₂ Ethylenediamine en Formulaarrow_forwardYou are comparing two coordination complexes that have the same molecular shape and central metal cation. Complex A has only chloride ligands and Complex B had only CO ligands. Which statements are true regarding the complexes? Select all that apply. Complex A has the smaller crystal field splitting. Light of higher frequency is required to promote an electron from the lower to the higher d orbitals in complex A relative to complex B. The light emitted as a photon is released to relax an electron from higher to lower energy d orbitals has a longer wavelength for complex A. Light of higher frequency is required to promote an electron from the lower to the higher d orbitals in complex B relative to complex A. The light emitted as a photon is released to relax an electron from higher to lower energy d orbitals has a longer wavelength for complex B. Complex B has the smaller crystal field splitting.arrow_forwardAn octahedral complex having the formula [M(dien)(NH3)(OH2)F] has a total of 15 isomers. In the sum formula “dien” represents the tridentate diethylenetriamine ligand, which can adopt meridional or facial coordination modes. Draw structures of 8 possible isomers, clearly showing any pairs of enantiomers. The tridentante ligand may be abbreviated as below for simplicity.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY