
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
4 NH3 + 5 O 2 -----> 4 NO + 6 H2O
HOW MANY MOLES OF OXYGEN (O2) ARE NEEDED TO REACT WITH 51.12 GRAMS OF AMMONIA(NH3) BY THIS REACTION ?
NH3 MOLAR MASS = 17.04g/mol
2 C4H10+13 O2 --------------> 8 CO2+ 10H2O
HOW MANY GRAMS OF WATER (H2O) ARE PRODUCED IF 100.0 MOLES OF C4H10 ARE USED?
H2O MOLAR MASS = 18.02 G /MOL
Expert Solution
arrow_forward
Step 1
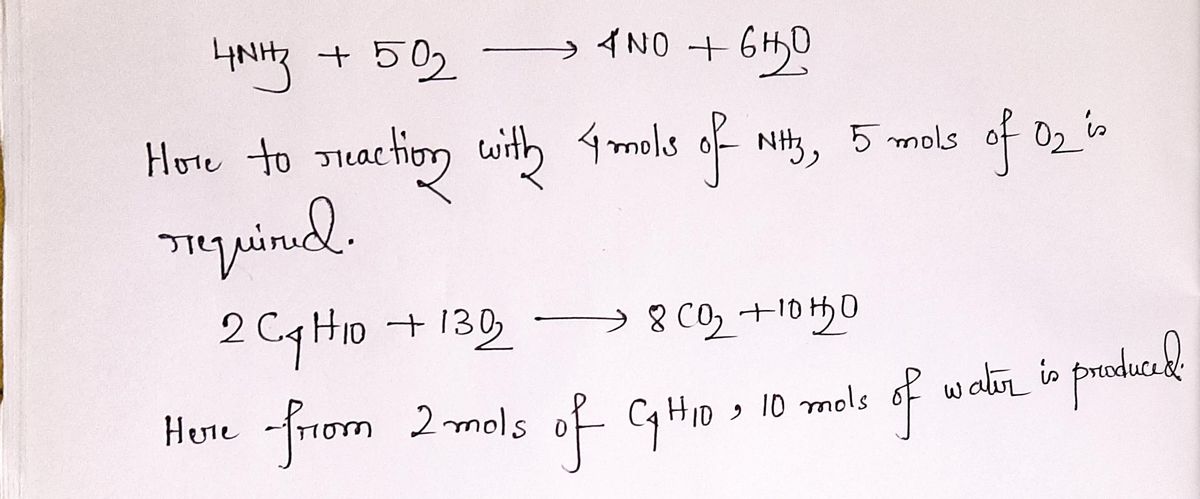
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What mass of oxygen (O2) is required to react completely with 25.0 g of C6H14? 2 C6H14 + 19 O2 → 12 CO2 + 14 H2Oarrow_forward4 HCI (aq) + MnO2 (s) → MnCl2 (aq) + 2 H,O (g) + Cl, (g) Chlorine gas can be made in the lab by the reaction of hydrochloric acid and manganese(IV) oxide, as shown in the reaction above. How many moles of chlorine can form from 6.75 moles of hydrochloric acid? 4 KNO3 2 K20 +2 N2 + 5 O2 Potassium nitrate decomposes upon heating to produce potassium oxide, nitrogen, and oxygen gases. How many moles of potassium nitrate need to be decomposed to produce 3.512 moles of oxygen?arrow_forwardConsider the following reaction: 2 C2H6O2 +5 02 +4 CO2 + 6 H2O What mass, in grams, of H2O is formed when 8.65 g of O2 reacts?arrow_forward
- 4. An old antacid commercial claimed that each tablet of their product could "neutralize 47 times its mass in stomach acid". The active ingredient in the antiacid tablet, NaAl(OH)₂CO3, reacts with HCl in stomach acid according to this balanced reaction: NaAl(OH)₂CO3 + 4 HCl →→ NaCl + AlCl³ + 3 H₂O + CO₂ How many moles of HCl can a 1.03 g of antiacid tablet neutralize if the tablet contains 0.246 g of the active ingredient? If stomach acid has a concentration of 0.14 M HCl, assuming the density of stomach acid to be similar to that of water (1.00 g/mL), what is the mass of stomach acid that the 1.03 g of antiacid tablet can neutralize? Does this number support the claim in the commercial?arrow_forwardBaking soda is a pure substance, sodium bicarbonate. Vinegar is a mixture of acetic acid dissolved in water, and it is only the acetic acid that reacts. Acetic acid and sodium bicarbonate can react together according to the chemical equation below: HC2H302 NaHCOe NAC2H302 + H20 + CO2 acetic acid + sodium bicarbonate sodium acetate + water + carbon dioxide Samuel wants to help his brother make a science fair volcano. 1. For his first experiment, Samuel obtains samples of solid acetic acid powder and solid sodium bicarbonate powder from his chemistry teacher. He mixes the two powders together, but there is no reaction. b) Considering the state of matter of the particles, give a possible explanation for why there is no reaction. BIUEE O Σ Next * Previous i Aa 2048 37.006 stv MacBook Air 888 F11 F10 80 FS F4 F3 F2 F1 + & @ 2# $ 6 7 8 2 3 4 Y Q E R G J K A S D F > V B M с command option command .. ·- > Narrow_forwardFor the reaction 3KOH+H3PO4⟶K3PO4+3H2O3KOH+H3PO4⟶K3PO4+3H2O how many grams of phosphoric acid, H3PO4, are needed to react completely with 13.513.5 g of potassium hydroxide, KOH?arrow_forward
- Lead(IV) oxide reacts with hydrochloric acid to give chlorine. The unbalanced equation for the reaction is PbO2 + Cl- -----> PbCl2 + Cl2 How many grams of PbO2 must react to give 30.1 g of Cl2?arrow_forward3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O In the above equation how many moles of water can be made when 178.7 grams of HNO3 are consumed?arrow_forwardConsider the reaction between ammonium and oxygen: 4 NH3 ) + 5 02 () --> 4 NO ) + 6 H20() A chemist allows 10.8 g of NH3 and 5.30 g of O2 to react. When the reaction is finished, the chemist collects 3.00 g NO. Determine the percent yield for the reaction.arrow_forward
- How many moles of ammonia would be required to react exactly with 0.576 moles of copper(II) oxide in the following chemical reaction? 2 NH₃(g) + 3 CuO(s) → 3 Cu(s) + N₂(g) + 3 H₂O(g) How many moles of nitrogen gas would be produced if 4.81 moles of copper(II) oxide were reacted with excess ammonia in the following chemical reaction? 2 NH₃(g) + 3 CuO (s) → 3 Cu(s) + N₂(g) + 3 H₂O(g) What is the mass in grams of CuO that would be required to produce 13.50 moles of nitrogen gas in the following chemical reaction? 2 NH₃(g) + 3 CuO(s) → 3 Cu(s) + N₂(g) + 3 H₂O(g) How many molecules of SO₃ can be formed from 0.18 moles of O₂ (assuming excess SO₂) from the following UNBALANCED equation? SO₂(g) + O₂(g) → SO₃(g)arrow_forwardConsider the following reaction. Na2CO3 + Mg(NO3)2 --> MgCO3 + 2NaNO3 Assuming the reaction goes to completion, when 25 mL of a 0.1 M Na2CO3 solution reacts with 22 mL of a 0.3 M Mg(NO3)2 solution, what is the maximum nuber of grams of MgCO3 that can produce? Which is the limiting reactant in the chemical reaction?arrow_forwardSodium Chloride reacts with lead II nitrate according to the following equation: NaCl + Pb(NO3)2 --> PbCl2 + 2NaNO3 If 4.10 grams of lead II nitrate reacts, how many grams of sodium nitrate is produced?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY