
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
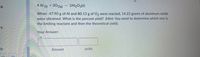
Transcribed Image Text:18:
4 Al (S) + 302(g)
2AI2O3(s)
When 47.90 g of Al and 80.13 g of O, were reacted, 14.22 grams of aluminum oxide
were obtained. What is the percent yield? (Hint: You need to determine which one is
the limiting reactant and then the theoretical yield).
9:
Your Answer:
CO:
Answer
units
Expert Solution
arrow_forward
Step 1
Aluminum reacts with oxygen to form the aluminum(III) oxide. The balanced chemical reaction is as follows:
Mass of aluminum reacted is = 47.90 g
The mass of oxygen reacted is = 80.13 g
The percent yield of the reaction is =?
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the reaction below produced 20.14 g of AlCl3 : Al2(CO3)3 + 6 HCl → 2 AlCl3 + 3 H2O + 3 CO2 If it was determined by stoichiometry that the reaction should produced 27.51 g of AlCl3. Determine the percent yield of the reaction (answer to 2 decimal places).arrow_forwardAl2Cl6 is made by treating scrap metal with chlorine gas according to the following equation: 2 Al (s) + 3 Cl2 (g) ⟶⟶ Al2Cl6 (s) In the reaction of 251.4 g Cl2 with 129.7 g Al, determine the limiting reactant and theoretical yield of the product. The limiting reactant is ?. The theoretical yield of Al2Cl6 is ? g. Round the answer to 1 decimal place. If the reaction produces 278.0 g Al2Cl6, calculate the percent yield of the reaction. Answer in % Round the answer to 1 decimal place.arrow_forwardGlucose (C6H12O6) is broken down in cells through a series of redox reactions ending with the reduction of oxygen to water and the release of carbon dioxide. The overall process is also known as respiration. The balanced equation is shown below: C6H12O6 (s) + 6O2 (g) → 6H2O (g) + 6CO2 (g) If 332 grams of glucose were consumed, how many grams of water would be breathed out? Assume an excess of oxygen gas. Molar mass of glucose: 180.16 g/molarrow_forward
- When propane (C3Hg) reacts with oxygen, carbon dioxide and water are produced. The balanced equation for this reaction is C3 Hs (g) + 502 (g) → 3CO2(g) + 4H2O(g) If 10 moles of oxygen react, The reaction consumes moles of propane (C3H8). The reaction produces moles of carbon dioxide and moles of water.arrow_forwardusing the equation: 2Al(s)+3Cl2(g)=Al2Cl6(s) , calculate the mass of Al required to produce 34.2g of Al2Cl6.arrow_forwardLiquid nitroglycerine (CaHsNaO₂) is a powerful explosive. When it detonates, it produces a gaseous mixture of nitrogen, water, carbon dioxide and oxygen. If 5.55 g of nitroglycerine explodes and 2.98 g of CO₂ is collected, what is the percentage yield of carbon dioxide?arrow_forward
- consider the following balanced equation 15O2(g)+2C6H5COOH(aq) ___ 14CO2(g)+6H2O(I) if 11.6 moles of O2(g) and 2.34 moles of C6H5COOH(aq) are allowed to react, and the percent yield is 76.7% how many moles of H2O(I) will actually be produced?arrow_forwardA sample of 4.00g of methane (CH4) is mixed with 15.0g of chlorine (Cl2). Determine which is the limiting reactant according to the following equation: CH4(g) + 4Cl2(g)> CCl4(e) + 4HCl(g)arrow_forwardAmmonia is produced by the balanced chemical reaction: 3H2lg) + N2g) → 2NH3(g) How many grams of ammonia can be produced from 22.7g of hydrogen with excess nitrogen present? (Hint: This is a limiting reagent question. Do not forget your significant digits and your units.) How many grams of ammonia can be formed from 36.3g of nitrogen with excess hydrogen present? (Hint: This is a limiting reagent question. Do not forget your significant digits and your units.) This is a limiting reactant problem: How many grams of ammonia can be produced from 22.7 g of hydrogen and 36.3 g of nitrogen? (Hint: This is a limiting reagent question. Do not forget your significant digits and your units. Also note that this question should be easy of you have answered Part A and Part B successfully.)arrow_forward
- [References] Ammonia gas can be prepared by the reaction of a metal oxide such as calcium oxide with ammonium chloride. CaO(s) + 2 NH, Cl(s) → 2 NH3 (g) + H. O(g) + CaCl, (s) If 102 g of CaO and 241g of NH, Cl are mixed, what is the limiting reactant, and what mass of NH3 can be produced? The limiting reactant is CaO Mass of NH3 = 6. Drag and drop your selection from the following list to complete the answer: NH3 NH, C1 CaCl, 23arrow_forwardUse the balanced equation, 4K + O2-->2K2O , to answer the following question. How many grams of O2 are needed to react completely with 16.6 g of K ? Be sure your answer has the correct number of significant figures.arrow_forwardIn a certain chemical reaction Compound A combines with Compound B to produce Compound C (and no other products). Measurements were taken of the amounts of A and B present before and after a reaction that produced some C: Compound initial amount final amount A 9.5 g 0 g 1.5 g 0.8 g Calculate the theoretical yield of C. Round your answer to the nearest 0.1 g. Suppose thẻ percent yield of C in this reaction was 55.%. Calculate the actual amount of C that was isolated at the end of the reaction. Round your answer to the nearest 0.1 g. Explañation Check O 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy Ace oloarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY