
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
The problem that is listed below need to be solved and you may access that problem via viewing them through the attached images in this request. **Question Number #3.53**
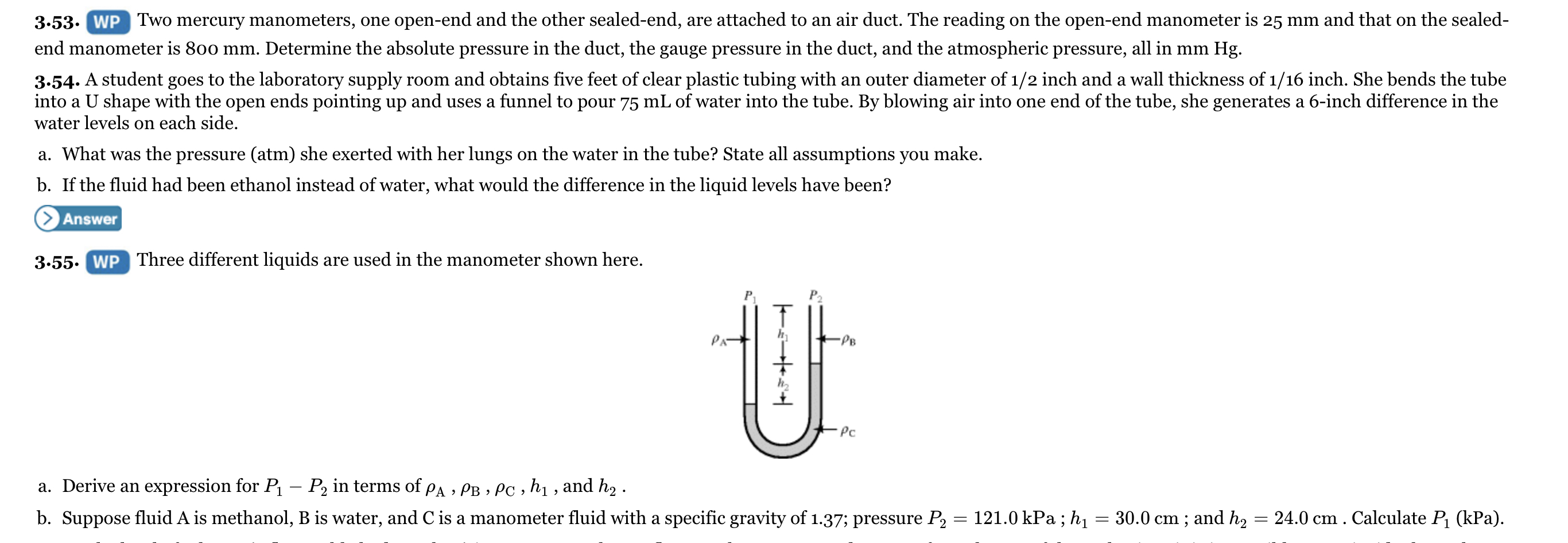
Transcribed Image Text:3.53. WP Two mercury manometers, one open-end and the other sealed-end, are attached to an air duct. The reading on the open-end manometer is 25 mm and that on the sealed-
end manometer is 800 mm. Determine the absolute pressure in the duct, the gauge pressure in the duct, and the atmospheric pressure, all in mm Hg.
3.54. A student goes to the laboratory supply room and obtains five feet of clear plastic tubing with an outer diameter of 1/2 inch and a wall thickness of 1/16 inch. She bends the tube
into a U shape with the open ends pointing up and uses a funnel to pour 75 mL of water into the tube. By blowing air into one end of the tube, she generates a 6-inch difference in the
water levels on each side.
a. What was the pressure (atm) she exerted with her lungs on the water in the tube? State all assumptions you make.
b. If the fluid had been ethanol instead of water, what would the difference in the liquid levels have been?
> Answer
3.55. WP Three different liquid
used in the manometer shown here.
P2
PA
•PB
Pc
a. Derive an expression for P – P2 in terms of pA » PB » PC , h1 , and h2 .
b. Suppose fluid A is methanol, B is water, and C is a manometer fluid with a specific gravity of 1.37; pressure P, = 121.0 kPa ; hị
= 30.0 cm ; and h2
24.0 cm . Calculate P (kPa).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 3) mycard: (The Front-End) • Create variables for five variables: name, accountnumber, unpaidbalance, newpurchases, and topay Ask for each of the first four variables Create a cardholder instance: cardholder bob=new cardholder(unpaid balance, newpurchases, name, accountnumber); Using getCurrentBalance(), getTotalBalance(), isActive(), print out (either using getters or a toString method): O Their name Their account status (active or frozen) 0 The current balance O The total balance Then ask how much they want to pay on their current balance (store in topay) and print out whether they are paid up for the month (good to go) or their unpaid balance has increased.arrow_forwardanswers given steps how to reach these pleasearrow_forwardPLEASE ANSWER QUESTION WITH RIGHT ANSWERS, ANSWERS ATTACHED FEEDBACK ATTACHED ALSO DONT COPY AND PASTE FROM OTHER ANSWERED QUESTIONS AS THESE ARE WRONGarrow_forward
- A Unit 3 Tutorial.pdf - Adobe Acrobat Reader DC (32-bit) File Edit View Sign Window Help Home Tools Process Co. Process Co. Unit 6 Part. Unit 6 Part. PRCCHA2 . PRCCHA2 . Test 2 Me. Unit 3 Tut. x Sign In 1 / 2 119% Question 2 Search 'Reduce Size' A 0.4 m³ vessel is used to store liquid propane at its vapour pressure. Safety considerations dictate that at a temperature of 320 K the liquid must occupy no more that 75 percent of the total volume of the vessel. For these conditions, determine the mass of the vapour in the vessel using the Redlich-Kwong equation of state. At 320 K the vapour pressure of propane is 16.0 bar. Export PDF Edit PDF Create PDF O Comment Data Tc = 369.8 K Pc = 42.5 bar R= 83.14 bar.cm³/(mol.K) i Combine Files Formula El Organize Pages v * Compress PDF 2 Redact A Prepare Form A Request E-signat. l Fill & Sign A Send for Comme. 19:29 P Type here to search 94 W 7°C ^ a O a a 4)) ENG 2022/05/08 21arrow_forwardI am having problems navigating. First of all, I signed up for this to get access to essays and now I cant find the essay i needarrow_forwardfeed back and answers attached to help with quesarrow_forward
- Nonearrow_forward3-19. Draw the block diagram representing the following transfer functions. In each case, do not do any algebraic manipulations to simplify the transfer functions, but use the rules of block diagram algebra to simplify the diagram if possible. (a) Y(s) = (b) (c) Ctrl Shift Tab Y(s) = Y₁(s) = Y₂ (s) CapsLk [XIDOLBY AUDIO Esc FnLock 3-20. Determine the transfer function C(s)/R(s) for the 5°C Mostly cloudy H Q Search BOUR Fn K₁ TIS + 1 1 2 Q A TS+1 = G₁(s) X (s) + G3 (s) Y2 (s) = G₂ (s) Y₁(s) @ Z 240 F W S Privacy Shutter on Webcam 360" Versatility Front Facing Speakers SO ·X (s) + ideapad Flex 5 4 [K₁ F1(s) - K₂ F2 (s)] 3+ 14+ # 3 X Alt E 220 K₂ T2S + 1 D CH C V X(s) QF+ 6 G Y B & 7 H U N 8 J M 9 F10 K O L Alt P Ctrlarrow_forwardG2 R (b) G1 H1 1 Given above block diagram, we know that H1 = 1, G1 = s+2 » G2 = ;, S please determine the transfer function C/R +arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The