
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
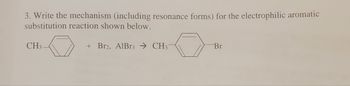
Transcribed Image Text:3. Write the mechanism (including resonance forms) for the electrophilic aromatic
substitution reaction shown below.
+ Br2, AlBr3 CH3-
CH3
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- b Assuming that halogens add to alkynes in the same manner that they add to alkenes, write a mechanism for the reaction step below. Use curved arrows to show electron reorganization. Arrow-pushing Instructions H3C C↔X ™ Br: CH3 Brarrow_forward7. The following scheme shows the intermediates in the mechanism for the synthesis of isoamyl acetate, an ester with a characteristic banana flavor. Classify steps 1, 2, 3, and 4 as addition, elimination, substitution, or acid base reactions. lo ОН gor H2SO4 2 HO HO QH₂ H2SO4 1 .H ОН له عليه ملا 4 HO 3 لیڈ جل OH HO OH + H2O Harrow_forwardi need help drawing the productarrow_forward
- Following is a balanced equation for the allylic bromination of propene. CH2==CHCH3 + Br2 h CH2==CHCH2Br + HBr (a) Calculate the heat of reaction, H 0, for this conversion. (b) Propose a pair of chain propagation steps and show that they add up to the observed stoichiometry. (c) Calculate the H 0 for each chain propagation step and show that they add up to the observed H 0 for the overall reaction.arrow_forwardChemistry question regarding type of reactionarrow_forwardCan you explain it? Provide examples with the various steps and explanations. How halogens affect the boiling/melting point? Does number of halogens affect it? For example, Ch3-Ch3 vs Ch3-Br vs Br-Ch3-Brarrow_forward
- First Writedown which reaction it is? SN1, SN2, E1? Write a stepwise mechanism for the following reactions showing ALL intermediates. Use curved arrows to symbolize the flow of electrons to show how each of the intermediates and product are formed. Show all necessary lone pairs and formal charges.arrow_forwardHighlight the electrophilic carbon in red, and highlight the leaving group in blue. Highlight the atom in the nucleophile that will attack the electrophilic center in green. Only atoms need to be highlighted and not the lone pairs or formal charges. O O − Clarrow_forward2. Provide an arrow pushing mechanism for the following reaction. Show all intermediate structures and formal charges. You do not need to show resonance structuresarrow_forward
- Rank these in order of increasing reactivity in an SN1 reactionarrow_forward4. A CHEM 245 student wants to synthesize some ethylene glycol to use as antifreeze in his radiator this winter. He proposes the following reaction to his instructor, who quickly explains that this reaction won't work as proposed due to the student's choice of reagent. 1) NaH 2) H20 OH Но a) Why can't sodium hydride (NaH) be used as the nucleophile in the reaction above? b) Propose an alternate reagent that COULD be used with the ethylene oxide to successfully give the desired ethylene glycol product.arrow_forward3. SN2 reactions are useful in the synthesis of many pharmaceutical compounds. The antiviral agent acyclovir can be prepared using an S№2 reaction as shown below followed by a hydrolysis reaction. The hydrolysis reaction will be discussed in CHEM 40B. R'RN A + B SN2 R'RN ELECTROPHILE: من مهمه NUCLEOPHILE: Identify which molecule (A or B) would serve as the electrophile and which would serve as the nucleophile in the S№2 reaction. Place your responses in the box below. Connections to biology The amino acid cysteine is one of the common building blocks of proteins that contains the thiol (SH) functional group. The reactivity of the thiol group allows researchers to connect various molecules to proteins, a process known as labeling. For example, it is possible to connect fluorescent probes that allow us to trace the fate of proteins in the cell. H SH O Cysteine amino acid within a protein chain sa acyclovir LG H₂N S OH H O Cysteine amino acid labeled with electrophilic probe…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning