
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
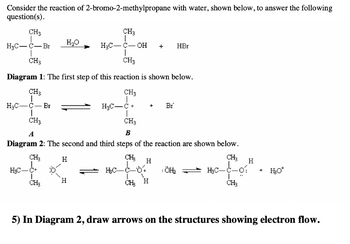
Transcribed Image Text:Consider the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following
question(s).
CH3
CH3
fo
H₂C-C-Br
CH3
CH3
Diagram 1: The first step of this reaction is shown below.
CH3
CH3
I
CH₂
H₂O
6
H₂C-C-Br
I
CH3
CH3
A
B
Diagram 2: The second and third steps of the reaction are shown below.
CH₂
H
H₂C-
H
OH +
H₂C-C
+ Br
H
H₂C-C-64
CH H
HBr
¡ÖH₂
CH₂
H₂C-C-01
L
CH₂
H
+
H₂O*
5) In Diagram 2, draw arrows on the structures showing electron flow.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Analyze the reaction below, and then provide the reagents necessary to carry out the conversion. Be sur with a brief explanation. он OH OHarrow_forwardRank compounds 1 – 4 (shown below) in terms of decreasing rates of cyclic ether formation. Use the provided ranking options.arrow_forwardComplete the following reactions and define which type of reactions are? 1- CH3CH2CHCICH2CH3 + H2O 2- CH3CH2CHCHCH¿CH3 + HI 3- CH3CHOHCH2CH3 + H2SO4 4- + H2O - он 5- + Cl2arrow_forward
- A common alkene starting material is shown below. Predict the major product for each reaction. Ignore any inorganic byproducts. I I Select to Select to Draw I Draw I HBr Cl₂ H₂O 1. BH3-THF 2. H2O2, NaOH nd 1.03 2. (CH3)2Sarrow_forwardneed help with this problem predict the major products. A-Carrow_forwardPick out the process which does not involve carbocation formation: O O ОН OH Br₂ CC14 HBr ether H₂SO H₂O HCI ether Br CI Br None of these involve a carbocationarrow_forward
- Draw a structural formula for the substitution product of the reaction shown below. ● H3C- ● H3C H Br + Na OCCH3 DMF • Use the wedge/hash bond tools to indicate stereochemistry where it exists. If more than one stereoisomer of product is formed, draw both. Separate multiple products using the + sign from the drop-down menu. Products that are initially formed as ions should be drawn in their neutral forms. DMF = dimethylformamidearrow_forwardComplete the following reaction. مله mom سله ه A B C D LiAlH4 ether B -CH2NH2 C -NH₂ D -OHarrow_forwardThe southern pine beetle utilizes a multi-component aggregation pheromone (one component shown below) to start mass colonization of healthy trees. The biosynthetic pathway involves the cyclization of this acetal from the straight chain structure. Draw the straight chain structure that could be used to form this acetal. Use wedges and dashes to correctly depict the stereochemistry. H3C- OH ✔arrow_forward
- Predict the major products of this organic reaction. Be sure to use wedge and dash bonds when necessary, for example to distinguish between different major products. c+ C :0 + T H₂SO4 2 но X 3 Click and drag to start drawing a structure.arrow_forward9.arrow_forward1. Draw the structure of sodium (E) 2-bromo-3-iodo-2-octenoate. 2. Give the IUPAC name of this compound, including stereochemistry. 3. Draw the most stable chais CH³arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY