
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Options for 1)increase or decrease
Options for 2)inverse or direct
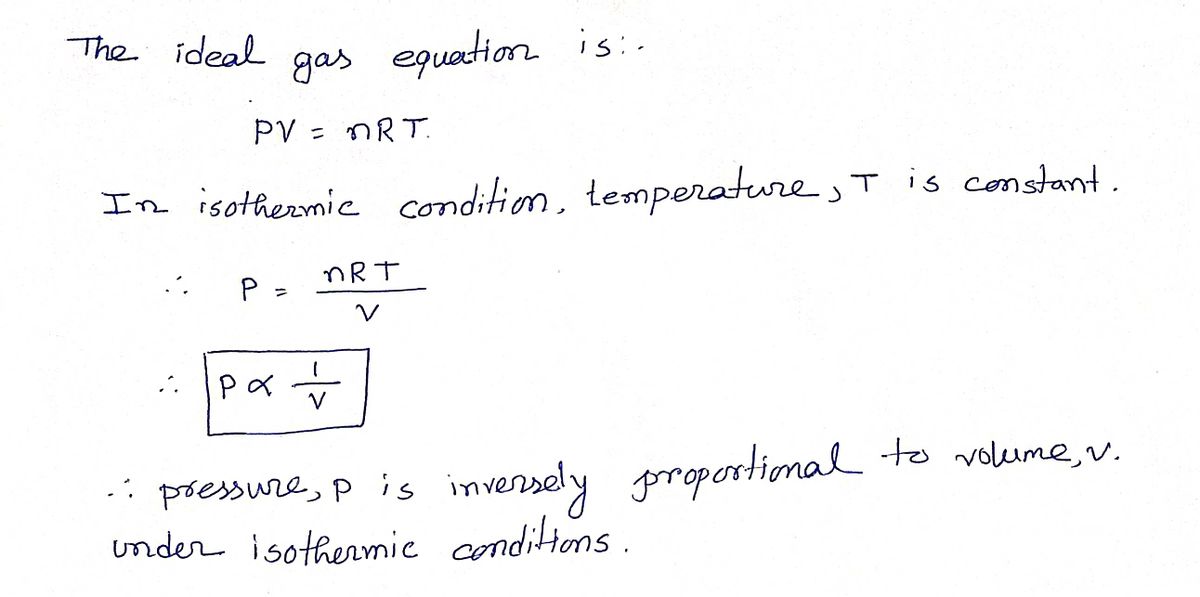
![3. When volume
of a gas
decreases under isothermic
conditions,
pressure
will
[ Select ]
This
is
a(n)
[ Select ]
relationship.
<>
<>](https://content.bartleby.com/qna-images/question/d6845ac8-a43c-4060-9850-941fe861469f/2ae97c94-7c7c-4276-839a-f679a75ca58e/0w0ms1a_thumbnail.jpeg)
Transcribed Image Text:3. When volume
of a gas
decreases under isothermic
conditions,
pressure
will
[ Select ]
This
is
a(n)
[ Select ]
relationship.
<>
<>
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 02A: 2.1-2.5 and Accuracy Handout (70 min) ulate Figures of Merit for Trueness ▾ Part A. Absolute Error A breathalyzer is a portable electrochemical instrument that measures the concentration of ethyl alcohol in a sample of exhaled breath A forensic chemist checks the calibration of a breathalyzer by testing a standard air sample known to contain 0.174 mg/L of ethanol seven times. The average value of these seven replicate measurements is found to be 0 157 mg/1 Calculate the absolute error of the breathalyzer. 1971 ΑΣΦΑ Submit Previous Answers Request Answer ? Previous x Incorrect; Try Again: 4 attempts remaining Q Search mgl ly Colle 16 of 17 E dx Next 11:44 PM 1/31/202 BANG &arrow_forwardent arch X Aktiv Chemistry ADD FACTOR x( ) 4.82 × 104 10¹2 A laser pulse is 4.82 x 10-2 milliseconds How many nanoseconds is this? 10-⁰ 4.82 x 10-8 10⁰ ns 10 º 4.82 × 10² X Question 30 of 31 4.82 x 10-14 ART ms PeopleSoft session expired 10-12 4.82 x 10-5 10⁰ ANSWER 4.82 x 1016 4.82 x 10-² 4.82 x 101⁰ min 1015 X ? Anthropology and Archaeolo X μs 10³ 4.82 × 10-11 RESET 5 10-3 P 1arrow_forwardThank you, but the answer is not accepted by the platform when I input it, I have tried calculating it but I get the same answer. What could be wrong?arrow_forward
- show final equation:arrow_forwardQuestion 1 of 20 A barrel of crude oil has a volume of 42 gallons, only approximately 45% of which is processed into gasoline. If your car achieves 32 mi/gal, and you drive 36,000 miles in one year, how many barrels of crude oil are required to run your car for a year? Tap here or pull up for additional resourcesarrow_forwardPerform the following calculations to the correct number of significant figures. [(2.33 x 10^6) / 42.370] + 132.99arrow_forward
- Often the analyst will compare known quantities of analyte to unknown quantities of the material to be analyzed. This may be done in one of three ways: -use calibration curves; use standard additions; or use internal standards. The method of standard additions would be used when A) the quantity of sample analyzed or the instrument response varies from run to run. B) the standard solutions and the unknown solution all have similar characteristics are unaffected by the other material in the sample. C) the sample composition is unknown or complex and affects the analytical signal.arrow_forwardSTARTING AMOUNT esc Tap here or pull up for additional resources ! 1 Q A X * 2 30² F2 W S # 3 80 F3 E D $ 4 (1000)¹ 0.01 100 Q F4 R ADD FACTOR * ( ) FL % 5 J F5 T m³ Convert 7.6 cm³ to m³ (0.1)* (0.01) 1000 (100)³ 7.6 x 10-4 G Question 25 of 31 ^ 6 cm³ F6 Y - ANSWER 0.076 m & 7 H (1)³ 7.6 cm K F7 * 00 0.1 7.6 x 10⁰ 8 RESET J 12 DII FB 1 1 ( 9 K F9 ) O O 4 F10 L Parrow_forwardSubmitting an external tool Available after May 9 at 11:59pm back I Review I Constants I P Part D [OH-] = 7.9 x 10-3 M Express your answer using two decimal places. pH: %3D Submit Request Answer MacBook Airarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY