
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
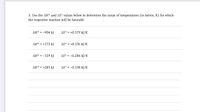
Transcribed Image Text:3. Use the AH° and AS° values below to determine the range of temperatures (in kelvin, K) for which
the respective reaction will be favorable
AH° = -904 kJ
AS° = +0.179 kJ/K
AH° = +173 kJ
AS° = +0.176 kJ/K
AH° = -139 kJ
AS° = -0.286 kJ/K
AH° = +285 kJ
AS° = -0.138 kJ/K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- = -93.1A]J/K Given the following information, calculate ArG° for the reaction below at 25 °C. Mg(s) + 1/2 02(g) → MgO(s) ArH° = -601.24 kJ/mol-rxn and ArS° = -108.36 J/Karrow_forwardGiven the standard heats of formation for the following compounds, calculate AH of the reaction at 298°. Fe304(s)_+ CO(g) → 3FEO(s) + CO2(g) AH J/mol) -1118 -110.5 -272 -393.5 O -263 kJ O 54 kJ O 19 kJ O -50 kJ 109 kJarrow_forwardfasdrfwadwarrow_forward
- What is AH° for the following reaction? 2C,H>(g) + 50,(g) → 4CO2(g) + 2H20(1) Substance AH®, (kJ/mol) C¿H>(g) +226.7 CO2(g) -393.5 H;0(!) -285.8 +2599.0 kJ -1692.2 kJ -452.6 kJ O -2599.0 kJ +1692.2 kJarrow_forwardFor the reactionI2(g) + Cl2(g) 2 ICl(g)G° = -30.7 kJ and S° = 11.4 J/K at 338 K and 1 atm.This reaction is (reactant, product) _______ favored under standard conditions at 338 K.The standard enthalpy change for the reaction of 2.05 moles of I2(g) at this temperature would be ______ kJ.arrow_forwardFor a particular reaction at 140.6 °C, AG = 560.53 kJ/mol, and AS = 131.79 J/(mol · K). Calculate AG for this reaction at -68.1 °C. AG = 467.18 kJ/mol Incorrectarrow_forward
- Hydrogen sulfide decomposes according to the following reaction: 2H2S(g) → 2H2(g) + S2(g) - = For this reaction at 298 K, AS rxn = 78.1 J/K mol, Arxn = 169.4 kJ/mol, and AG rxn 146.1 kJ/mol. What is AG rxn at 900. K?arrow_forwardFor the reaction 4HCI(g) + O2(g) 2H20(g) + 2C12(g) AH° = -114.4 kJ and AS° = -128.9 J/K The equilibrium constant for this reaction at 310.0 K is Assume that AH° and AS° are independent of temperature.arrow_forwardFor a particular reaction at 128.2 °C, AG = 190.07 kJ/mol, and A.S = 658.97 J/(mol K). Calculate AG for this reaction at 21.3 °C. kJ/mol AG =arrow_forward
- A Mo another question will save this response. stion 16 Consider the following reaction at 25°C if PpcIs=0.0029 atm, PpCi3 =0.27 atm, and PC12= 0.40 atm PC13 (0 + Cl2 (g)- PCl, (g) AH°, = -0.1702 kJ/K What is the value of O? 0.02685 What is the value of AG? -37.2KJ Is the reaction spontaneous under these conditions? Yes, the reaction is spontanerarrow_forwardA certain reaction has ∆?°= -19.5KJ and ∆S°= 42.7 J/K, is the reaction exothermic or endothermic?arrow_forwardConsider the following reaction at 25 °C: 3 Ni(s) + N2(g) + 3 H20(g) → 3 NiO(s) + 2 NH3(g) Given the information in the table, calculate AH° for the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY