
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
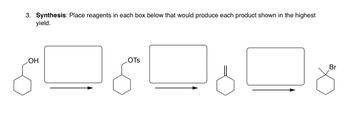
Transcribed Image Text:**Synthesis Problem:**
**Instructions:** Place reagents in each box below that would produce each product shown in the highest yield.
**Diagram Details:**
1. **Reaction 1:**
- **Starting Material:** Cyclohexanol (indicated by the hexagonal ring with an -OH group attached).
- **Product:** Cyclohexyl tosylate (indicated by the hexagonal ring with an -OTs group attached).
- **Box:** Insert the reagent(s) needed to convert cyclohexanol to cyclohexyl tosylate.
2. **Reaction 2:**
- **Starting Material:** Cyclohexyl tosylate (indicated by the hexagonal ring with an -OTs group).
- **Product:** Cyclohexene (indicated by the hexagonal ring with a double bond).
- **Box:** Insert the reagent(s) needed to convert cyclohexyl tosylate to cyclohexene.
3. **Reaction 3:**
- **Starting Material:** Cyclohexene (indicated by the hexagonal ring with a double bond).
- **Product:** Bromocyclohexane (indicated by the hexagonal ring with a -Br group attached).
- **Box:** Insert the reagent(s) needed to convert cyclohexene to bromocyclohexane.
These transformations illustrate common reactions in organic synthesis, such as the conversion of alcohols to tosylates, elimination reactions to form alkenes, and halogenation of alkenes to form alkyl halides.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- C Show the complete mechanism and the product formed. AlCl3 ?arrow_forwardshow the major product/s of the given reactionarrow_forwardFor the reaction shown below, circle the major product obtained when the | reaction is allowed to reach equilibrium. Place a box around the product that is formed the fastest. а H2C= CI H-CI CIH2C CIH,C H3C CI CI `CH3 H2C CIH,C H;C CIarrow_forward
- What is the major organic product formed in the following sequence of reactions? A, B, C, or D?arrow_forward4) Show the most likely product(s) for each step in the reaction sequence shown below. Br Product C Mg diethyl ether 1) CO2 2) H3O+ Product C ㄷ Product Darrow_forward9. Complete Reactions. Provide only the major product for E2&SN2 reactions. E1/SN1 provide all possible products. NaOH Br provide mechanism CI Provide mechanism + CI NaOCH 3 KCNarrow_forward
- 4. Draw the final major product(s) for each multistep synthesis reaction. H3O* OMe H3PO4 Jones Et,NH reagent H30* PBR3 KOC(CH3)3 1. O3 NABH3CN HO- 2. DMS NH3, H3O* Br KOC(CH3)3 1. ВНз PCC H3O* 2. H2О2, ОН CH2CI2arrow_forward5. Predict the major product in the following reactions. MeO. CHO heatarrow_forwardPlease don't use hend raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY