
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Choose reagents to convert 2-cyclohexenone to the following compounds.
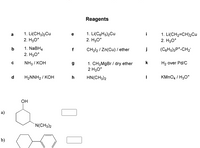
Transcribed Image Text:Reagents
1. Li(CH3)2Cu
2. H30*
1. Li(CgHs)2Cu
2. H30*
1. Li(CH2=CH)2Cu
2. H30*
a
i
1. NaBH4
f
CH212 / Zn(Cu) / ether
j
(CgHs)3P*-CH2
2. H30*
NH3 / KOH
1. CH3M9B / dry ether
2 H30*
k
H2 over Pd/C
d
HaNNH2/ КОН
h
HN(CH3)2
KMNO4 / H3O*
Он
a)
`N(CH3)2
b)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please explain how the product shown is formed from the starting materials and reagents H+ N NaBH3CN EtOH XXarrow_forwardThe following series of reactions will yield 2) CI-CH2-CH2-C6H5 1) CH3CH2SH + N2OH O 2-chloro-4-ethyl-benzenesulfonic acid O CH;CH2-S-CH2CH2C6H5 O ortho-chloro-ethyl-benzene O 1-phenyl-3-chloro-propane O CH3CH2-O-CH2CH2C6H5arrow_forwardDraw the major organic product when each of the below reagent is added to 3,3-dimethylbutene.arrow_forward
- Which one of the following compounds give racemic mixture when treated with NaOH (aq)? 1-Bromobutane 2-Bromo-2-methylpropane 2-Bromopropane 2-Bromobutanearrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. Draw a structural formula for the enol form of the carbonyl compound below.arrow_forwardIf phenoxide ion is allowed to react with 1-bromopentane, pentyl phenyl ether is obtained. However, if cyclohexane is used as the alkyl halide, the major products are phenol and cyclohexene. Explain how these products were formed.arrow_forward
- Draw the structure of the starting material needed to make 2-methylhept-3-yne using sodium amide in liquid ammonia, followed by 1-bromopropane. The starting hydrocarbon must have no more than five carbons 1) NaNH2, NH3() NaBr + 2) CHзCH2CH2Brarrow_forwardHow to synthesise 1-cyclohexylcyclohexanol from cyclohexanone?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY