
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
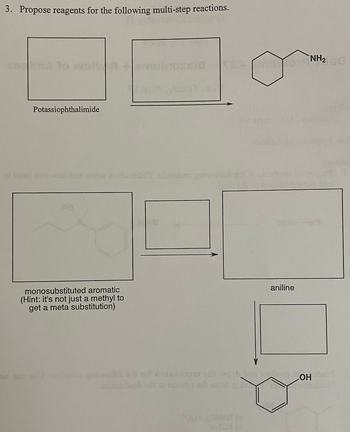
Transcribed Image Text:3. Propose reagents for the following multi-step reactions.
Potassiophthalimide
monosubstituted aromatic
(Hint: it's not just a methyl to
get a meta substitution)
aniline
NH20
than to OH
O.HOM (
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 3. Geef alle mogelijke reactieproducten die kunnen ontstaan wanneer je (1R, 2R,3S)-2-bromo-1-ethyl-3-methylcyclohexaan laat reageren met het ethoxide ion. Let op de juiste stereochemie en geef het reactiemechanisme.arrow_forwardThe following reaction will not occur as writtenarrow_forward1. CH3 Ph oto KOH O OH Ethanol Ph Ph-H -CH₂ + KCI H₂O 2. OCOH a = Proton transfer d Radical chain addition g= E2 Elimination b= Lewis acid/base e Electrophilic addition h = SN1 Nucleophilic substitution c = Radical chain substitution f = E1 Elimination i = SN2 Nucleophilic substitution Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1. 2. HCIarrow_forward
- 2 ok 5 Assignment - Carbonyl Chemistry Part II i M Question 22 - H.W. 6 Assignment - Carbonyl Chemistry Part II - Connect Step 5: O O H RO) RO O H H H H. H 90 H. JOY H 2 attempts left APR 11 H HIN Check my work M 4 < Prev ezto.mheducation.com 42 Saved Next part 22 of 30 2 JAarrow_forward1. H3C H CH3 2. H3C + Br CH3 H3C H excess NH3 NH4CI H H NH2 C2H5 N-C₂H5 C2H5 + C2H5 H-N-C2H5 Br C2H5 a Proton transfer b Lewis acid/base e Electrophilic addition h = Syl Nucleophilic substitution f El Elimination i = SN2 Nucleophilic substitution c Radical chain substitution g E2 Elimination j Electrophilic aromatic substitution d Radical chain addition Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - j for your answers.arrow_forwardte Bb Welcome, Kawtha... Maps News Home GE [Review Topics] [References] 1. Br H,SO, /H,O H20 Mg2+ Br HSO, 2. OH Na HCO, O Na CO2 H20 h = Sy1 Nucleophilic substitution i= SN2 Nucleophilic substitution- j= Electrophilic aromatic substitution a = Proton transfer e = Electrophilic addition b = Lewis acid/base f= El Elimination c = Radical chain substitution d = Radical chain addition g = E2 Elimination Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - j for your answers. 2. Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next Email Instructor Save and Exi Cengage Learning | Cengage Technical Supportarrow_forward
- Solve 65 66 67arrow_forward3. Geef alle mogelijke reactieproducten die kunnen ontstaan wanneer je (1R, 2R,3S)-2-bromo-1-ethyl-3-methylcyclohexaan laat reageren met het ethoxide ion. Let op de juiste stereochemie en geef het reactiemechanisme.arrow_forward17 eBook Print References Specify reagents suitable for converting 3-ethyl-2-pentene to 2,3-epoxy-3-ethylpentane. Select the single best answer. peroxyacetic acid 1. B₂H6; 2. H₂O2, HO™ 3 attempts left Check my work O H₂O, H₂SO4 Cr₂0₂², H 03, CH3OHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY