
Biology 2e
2nd Edition
ISBN: 9781947172517
Author: Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:### Transcription
**Question 3:**
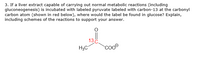
If a liver extract capable of carrying out normal metabolic reactions (including gluconeogenesis) is incubated with labeled pyruvate, labeled with carbon-13 at the carbonyl carbon atom (shown in red below), where would the label be found in glucose? Explain, including schemes of the reactions to support your answer.
**Diagram Explanation:**
The diagram shows a structural formula of pyruvate with the carbon-13 isotope label indicated in red at the carbonyl carbon:
- **Chemical Structure:**
- Pyruvate is shown with a methyl group \((\text{CH}_3)\), a carbonyl group \((\text{13C}=O)\), and a carboxylate ion (\(\text{COO}^-\)).
- The carbon-13, labeled in red, is part of the carbonyl group, which is the central carbon atom in the pyruvate structure.
When discussing the metabolic pathway, you should consider how gluconeogenesis incorporates this labeled carbon as pyruvate is converted into glucose, and which specific carbon in glucose will retain the label. Include reaction schemes of gluconeogenesis to illustrate the path and final position of the labeled carbon.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Figure 27.3 illustrates the response of R (ATP-regenerating) and U (ATP-utilizing) enzymes to energy charge. a. Would hexokinase be an R enzyme or a U enzyme? Would glutamine: PRPP amidotransferase, the second enzyme in purine biosynthesis, be an R enzyme or a U enzyme? b. If energy charge = 0.5: Is the activity of hexokinase high or low? Is ribose-5-P pyrophosphokinase activity high or low? c. If energy charge = 0.95: Is the activity of hexokinase high or low? Is ribose-5-P pyrophosphokinase activity high or low?arrow_forwardBoth prokaryotic and eukaryotic organisms carry out some form of glycolysis. How does ha fact support or not support the assertion that glycolysis is one of the oldest metabolic pathways?arrow_forwardUsing the ActiveModel for aldose reductase, describe the structure of the TIM barrel motif and the structure and location of the active site.arrow_forward
- Using the ActiveModel for enoyl-CoA dehydratase, give an example of a case in which conserved residues in slightly different positions can change the catalytic rate of reaction.arrow_forwardIs aerobic respiration more or less efficient than glycolysis? Explain your answer.arrow_forwardMATHEMATICAL What yield of ATP can be expected from complete oxidation of each of the following substrates by the reactions of glycolysis, the citric acid cycle, and oxidative phosphorylation? (a) Fructose-1,6-bisphosphate (b) Glucose (c) Phosphoenolpyruvate (d) Glyceraldehyde-3-phosphate (e) NADH (f) Pyruvatearrow_forward
- The Energetic Cost of Nitrogen Excretion via the Urea Cycle How many ATP equivalents are consumed in the production of 1 equivalent of urea by the urea cycle?arrow_forwardThe Reactions and Meehanisms of the Leloir Pathway Write the reactions that permit galactose to be utilized in glycolysis. Write a suitable mechanism, tor one of these reactions.arrow_forwardUnderstanding Enzyme Mechanisms Related to Pyruvate Carboxylase Based on the mechanism for pyruvate carboxylase (Figure 22.3), write reasonable mechanisms for the reactions that follow:arrow_forward
- Explain how glucose is metabolized to yield ATP.arrow_forwardThe Energy Cost of dTTP Synthesis (Integrates with Chapter 20.) Starting from HCO3, glutamine, aspartate, and ribosc-5-P, how many ATP equivalents are consumed in the synthesis of dTTP in a eukaryotic cell, assuming dihydroorotate oxidation is coupled to oxidative phosphorylation? How does this result compare with the ATP costs of purine nucleotide biosynthesis calculated in problem 2?arrow_forwardKetone bodies are used as an alternative source of fuel during starvation. Describe how ketones are synthesized.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning