
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
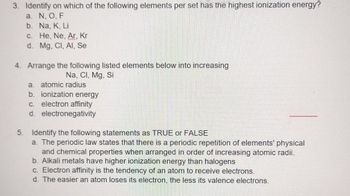
Transcribed Image Text:3. Identify on which of the following elements per set has the highest ionization energy?
a. N, O, F
b.
Na, K, Li
c.
He, Ne, Ar, Kr
d. Mg. Cl, Al, Se
4. Arrange the following listed elements below into increasing
Na, CI, Mg, Si
a. atomic radius
b. ionization energy
c. electron affinity
d. electronegativity
5. Identify the following statements as TRUE or FALSE
a. The periodic law states that there is a periodic repetition of elements' physical
and chemical properties when arranged in order of increasing atomic radii.
b. Alkali metals have higher ionization energy than halogens
c. Electron affinity is the tendency of an atom to receive electrons.
d. The easier an atom loses its electron, the less its valence electrons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Fluoride ion, F, has no unpaired electrons. Vanadium forms four binary fluoridesVF2, VF3, VF4, and VF5. Assume that all four are ionic compounds. (a) Which fluoride is diamagnetic? (b) Which fluoride has the greatest attraction to a magnetic field? (c) Which fluoride has two unpaired electrons per vanadium?arrow_forward6.80 Describe how valence electron configurations account for some of the similarities in chemical properties among elements in a group.arrow_forwardWhich set of ions are isoelectronic in their ground-state electron configurations?a. N, O, F, Ne b. Na+, K+, Rb+, Cs+ c. F–, Cl–, Br–, I–d. Mg2+, Ca2+, Sr2+, Ba2+ e. N3–, O2–, Mg2+, Al3+arrow_forward
- Which should have the largest difference between the first and second ionization energy-C, Li, N, or Be? c. d. Which has the largest ionization energy-Be, Mg, or Ca? Which of the following has the largest radius-s, CI, or K+? е.arrow_forwardThe following are isoelectronic species: N³-, F¯, and Mg²+. Rank them in order of increasing 2+ a. size. b. ionization energy. < c. electron attachment enthalpy. < Drag and drop your selection from the following list to complete the answer: Mg²+ N³- F-arrow_forwardNonearrow_forward
- Which atom is more likely to have the greater electron affinity (keep and take electrons)? F or Na? Cl or Mg? F or O? Cl or K?arrow_forwardAtoms of an element X have the ground-state electron configuration 1s22s22p63s23p4. Whattype of ion is X most likely to form?a. X6+ b. X3– c. X4+ d. X– e. X2–arrow_forwardFor each of the following ions, identify the neutral atom that has the same number of electrons. A. Zn2+ B. Sn4+ C. Sc.3+arrow_forward
- Which of these are isoelectronic with krypton? Circle all that qualify. a. Ca+2 b. Br-1 c. Sr+2 d. I-1 e. Ca+2 f. K+1 g. Rb+1 h. Zr+2 i. Zr+4arrow_forward6a. Which type of ions – cations or anions – have smaller radii than the corresponding neutral atoms? Explain why the ion radii are larger. b. Which would be smaller? Li or Li+? Explain why. c. Which would be smaller? Cl or Cl-? Explain why. d. Write the complete electron configuration for Cl atom. Please answer all parts of question.arrow_forwardWhich of the following statements is wrong? A. Rb has the largest atomic radius among Li, Na, K, and Rb. B. Mg+2 has the largest iconic radius among N3-, O2-, F-, Na+, and Mg2+ ions. C. The He atom shows the largest first ionization energy amon He, Ne, Ar, and Xe. D. The radius of F- is larger than that of F.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning