
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
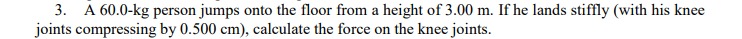
Transcribed Image Text:3. A 60.0-kg person jumps onto the floor from a height of 3.00 m. If he lands stiffly (with his knee
joints compressing by 0.500 cm), calculate the force on the knee joints.
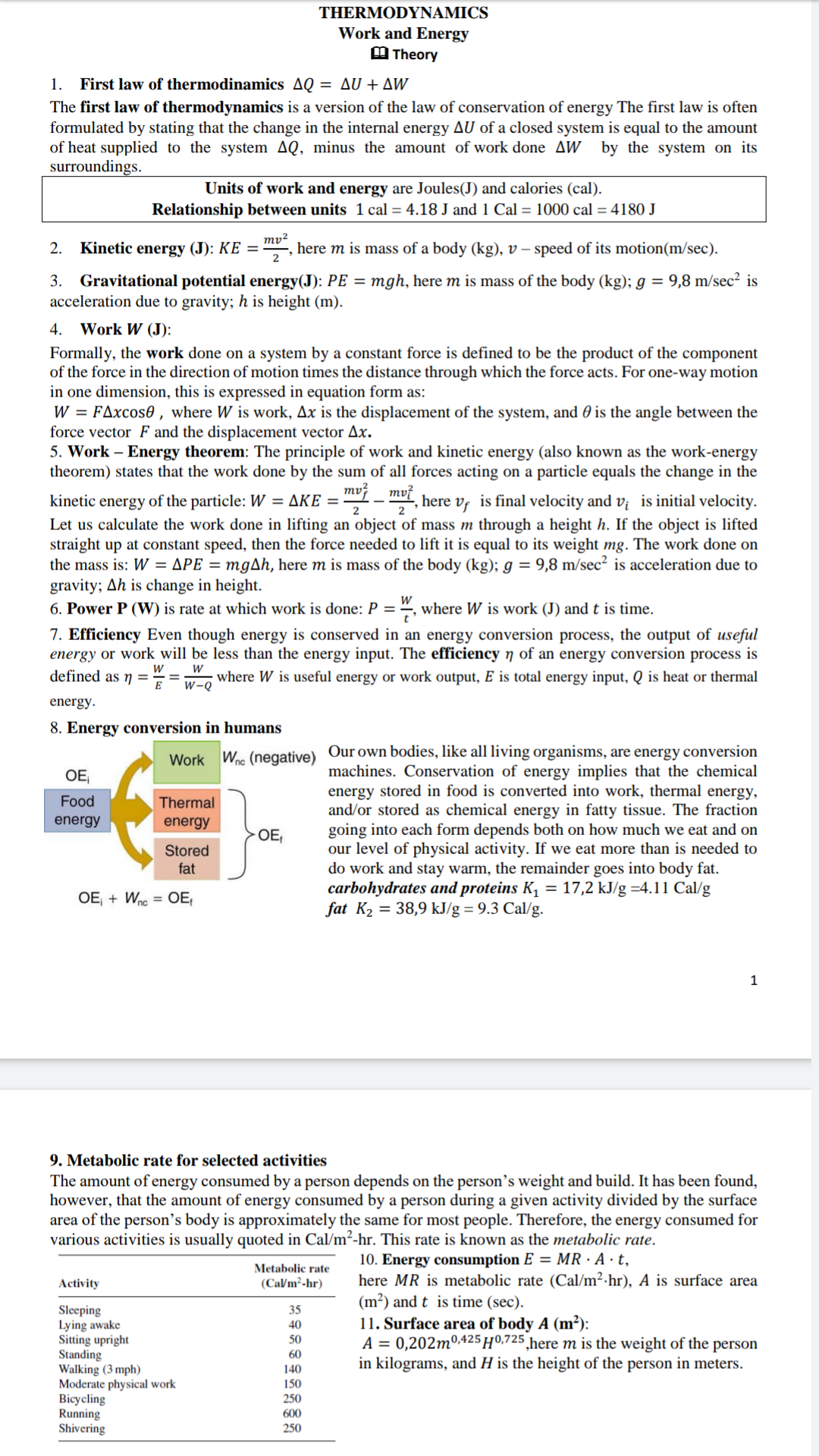
Transcribed Image Text:THERMODYNAMICS
Work and Energy
M Theory
1. First law of thermodinamics AQ = AU + AW
The first law of thermodynamics is a version of the law of conservation of energy The first law is often
formulated by stating that the change in the internal energy AU of a closed system is equal to the amount
of heat supplied to the system AQ, minus the amount of work done AW by the system on its
surroundings.
Units of work and energy are Joules(J) and calories (cal).
Relationship between units 1 cal = 4.18 J and 1 Cal = 1000 cal = 4180 J
ту?
here m is mass of a body (kg), v – speed of its motion(m/sec).
Kinetic energy (J): KE =
2
2.
3. Gravitational potential energy(J): PE = mgh, here m is mass of the body (kg); g = 9,8 m/sec? is
acceleration due to gravity; h is height (m).
4. Work W (J):
Formally, the work done on a system by a constant force is defined to be the product of the component
of the force in the direction of motion times the distance through which the force acts. For one-way motion
in one dimension, this is expressed in equation form as:
W = FAxcos0 , where W is work, Ax is the displacement of the system, and 0 is the angle between the
force vector F and the displacement vector Ax.
5. Work – Energy theorem: The principle of work and kinetic energy (also known as the work-energy
theorem) states that the work done by the sum of all forces acting on a particle equals the change in the
mv?
kinetic energy of the particle: W = AKE = "- , here v, is final velocity and v¡ is initial velocity.
Let us calculate the work done in lifting an object of mass m through a height h. If the object is lifted
straight up at constant speed, then the force needed to lift it is equal to its weight mg. The work done on
the mass is: W = APE = mgAh, here m is mass of the body (kg); g = 9,8 m/sec² is acceleration due to
gravity; Ah is change in height.
6. Power P (W) is rate at which work is done: P = ", where W is work (J) and t is time.
7. Efficiency Even though energy is conserved in an energy conversion process, the output of useful
energy or work will be less than the energy input. The efficiency 7 of an energy conversion process
defined as ŋ =
is
where W is useful energy or work output, E is total energy input, Q is heat or thermal
w-Q
energy.
8. Energy conversion in humans
Our own bodies, like all living organisms, are energy conversion
machines. Conservation of energy implies that the chemical
energy stored in food is converted into work, thermal energy,
and/or stored as chemical energy in fatty tissue. The fraction
going into each form depends both on how much we eat and on
our level of physical activity. If we eat more than is needed to
do work and stay warm, the remainder goes into body fat.
carbohydrates and proteins K, = 17,2 kJ/g =4.11 Cal/g
fat K2 = 38,9 kJ/g = 9.3 Cal/g.
Wne (negative)
Work
OE,
Food
Thermal
energy
energy
OE,
Stored
fat
OE + Wne = OE,
9. Metabolic rate for selected activities
The amount of energy consumed by a person depends on the person's weight and build. It has been found,
however, that the amount of energy consumed by a person during a given activity divided by the surface
area of the person's body is approximately the same for most people. Therefore, the energy consumed for
various activities is usually quoted in Cal/m²-hr. This rate is known as the metabolic rate.
10. Energy consumption E = MR · A · t,
here MR is metabolic rate (Cal/m²·hr), A is surface area
(m²) and t is time (sec).
11. Surface area of body A (m²):
A = 0,202m0425 H0,725 ,here m is the weight of the person
in kilograms, and H is the height of the person in meters.
Metabolic rate
(Ca/m²-hr)
Activity
Sleeping
Lying awake
Sitting upright
Standing
Walking (3 mph)
Moderate physical work
Bicycling
Running
Shivering
35
40
50
60
140
150
250
600
250
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Similar questions
- 4. A wooden board of mass M and length L is hung from the ceiling by two massless wires attached to its two ends. A block of mass m is placed on the board. The two wires are of the same length. (a) the two wires is equal to each other? (b) along the board? (c) other. The thick wire can just sustain the maximum tension in part (b) while the thin wire can only sustain half of it. What is the requirement on the position of the block to avoid breaking the wires? Where is the block placed if it is known that the tension in What is the minimum and maximum of tension in the wires as we move the block Now assume M = 10 kg, m = 15 kg, and that one of the wires is thinner than thearrow_forwardL GAS A 48.0 kg sign hangs at the end of a bar where L = 3.10 meters in length. A cable attaches to the end of the horizontal bar and to a wall 2.60 meters above where the bar is attached to the wall. The bar has a mass of 12-kg. What is the tension in the cable? Give your answer in Newtons to 3 significant figures (no decimal places in this case).arrow_forwardTo strengthen his arm and chest muscles, a 79-kg athlete 2.0 m tall is doing a series of push-ups as shown in the figure below (Eigure 1). His center of mass is 1.15 m from the bottom of his feet, and the centers of his palms are 30.0 cm from the top of his head. Figure -2.0m 1 of 1 30.0 cm Find the force that the floor exerts on each of his feet, assuming that both feet exert the same force. Express your answer in newtons. F= Submit Part B F= [5] ΑΣΦ Submit Request Answer Find the force that the floor exerts on each of his hands, assuming both palms exert the same force. Express your answer in newtons. 1971 ΑΣΦ Request Answer ? N Narrow_forward
- When you lift an object by moving only your forearm, the main lifting muscle in your arm is the biceps. Suppose the mass of a forearm with hand is 1.60 kg. If the biceps is connected to the forearm a distance of 2.4 cm from the elbow, how much force must the biceps exert to hold a 42 N ball at the end of the forearm at distance of 36.0 cm from the elbow, with the forearm parallel to the floor, in Newtons? Use g = 10.0 m/s2. Your answer needs to have 3 significant figures, including the negative sign in your answer if needed. Do not include the positive sign if the answer is positive. No unit is needed in your answer, it is already given in the question statement.arrow_forwardA meter stick is supported by a knife-edge at the 50-cm mark. You hang masses of 0.40 kg and 0.60 kg from the 20-cm and 80-cm marks, respectively. Where should you hang a third mass of 0.30 kg to keep the stick balanced?arrow_forwardWhen you lift an object by moving only your forearm, the main lifting muscle in your arm is the biceps. Suppose the mass of a forearm with hand is 1.60 kg. If the biceps is connected to the forearm a distance of 2.1 cm from the elbow, how much force must the biceps exert to hold a 35 N ball at the end of the forearm at distance of 36.0 cm from the elbow, with the forearm parallel to the floor, in Newtons? Use g = 10.0 m/s2. Your answer needs to have 3 significant figures, including the negative sign in your answer if needed. Do not include the positive sign if the answer is positive. No unit is needed in your answer, it is already given in the question statement.arrow_forward
- A tutor told me that the answer to 1b was 10N but I don't understand why it would be 10N for the tension. If that is correct could someone break it down for me?arrow_forward3. A large board L 6.00 m and m 25.0 kg is laying so that d = 1.00 m of the board hangs off the roof of a building. A contractor puts his toolbox on the end of the board as shown. What is the greatest mass the toolbox can have and not cause the board to flip up and slide off the roof?arrow_forward1:51 kık- M A A .content.blackboardcdn.com 5 46 Chapter 2 Problems 2.14 A box weighing 25 pounds (assumed concentrated at its center of gravity) is being pulled by a horizontal force F equal to 20 pounds. What is the moment about point A? Does the box tip over? 2.15 A large wood beam weighing 800 N is supported by two posts as shown. If an unthinking man weighing 700 N were to walk on the overhang portion of the beam, how far can he go from point A before the beam tips over? (Assume the beam is resting on the two supports with no physical connection.)arrow_forward
- For a meter stick of mass 147.89 g, it is found that its weight can be balanced by hanging a 183.99 g mass at the 95.00 cm mark. Where does the center of the weight of the meter stick locate on the meter stick? Enter the number in cm unit.arrow_forwardWhen you lift an object by moving only your forearm, the main lifting muscle in your arm is the biceps. Suppose the mass of a forearm with hand is 1.60 kg. If the biceps is connected to the forearm a distance of 2.4 cm from the elbow, how much force must the biceps exert to hold a 42 N ball at the end of the forearm at distance of 36.0 cm from the elbow, with the forearm parallel to the floor, in Newtons? Use g = 10.0 m/s2. Your answer needs to have 3 significant figures, including the negative sign in your answer if needed. Do not include the positive sign if the answer is positive. No unit is needed in your answer, it is already given in the question statement.arrow_forwardThe 600-kg uniform I-beam supports the load shown. Determine the reactions at the supports. Answers: Ax Ay = = By = p -7.5 m- +4.5 m 285 kg N N N Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON