
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
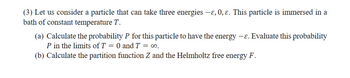
Transcribed Image Text:(3) Let us consider a particle that can take three energies —ɛ, 0, ɛ. This particle is immersed in a
bath of constant temperature T.
(a) Calculate the probability P for this particle to have the energy -ε. Evaluate this probability
P in the limits of T = 0 and T = ∞o.
(b) Calculate the partition function Z and the Helmholtz free energy F.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A monatomic particle at rest can be in either of two energy levels, separated by an energy ε. Consider a dilute gas of N such particles at a fixed temperature T. a) Write down the probability for a single particle to be moving with momentum h❘k and in the excited energy level. b) Derive the canonical partition function of a single particle. c) [You may use the Gaussian integral: ୮ dx x²e-x²y² = 4y3 and approximate the density of states in three dimensions as g(k)dk ≈ k²dk] Hence, or otherwise, find the internal energy per particle of this gas and comment on how it behaves at large temperaturearrow_forwardShow that at high enough temperatures (where KBT » ħw) the partition function of a simple quantum mechanical harmonic oscillator is approximately Z≈ (Bħw)-¹ Then use the partition function to calculate the high temperature expressions for the internal energy U, the heat capacity Cy, the Helmholtz function F and the entropy S.arrow_forwardThe partition funetion for the ensemble characterized by constant V, E, and G = µÑ is given to a very good approximation by ø(V, E, µN)=Q(N,V,E)eBHN, where G = µN is the Gibbs energy (µ is the chemical potential and N is the average number of particles). Find an expression for the characteristic thermodynamic function for this ensemble in terms of the partition function ø(V, E, µN).arrow_forward
- The energy spectrum of a particle consists of four states with energies 0,e, 2 e,3 e. Let Z, (T),Z, (T) and Ze(") denote the canonical partition functions for four non- interacting particles at temperature T. The subscripts B, F and C corresponds to bosons, fermions and distinguishable classical particles, respectively. Let y= exp k„T Which one of the following statements is true about ZB (T), ZF (T) and Zc (T)? (a) They are polynomials in y of degree 12,6 and 12, respectively. (b) They are polynomials in y of degree 16,10 and16, respectively (c) They are polynomials in y of degree 9,6 and 12, respectively. (d) They are polynomials in y of degree 12,10 and 16, respectively.arrow_forwardObtain the expression for the average thermal energy when the partition function is Z: ħo (A) 극청고 2 (B) kzT 1 (C) -k,T 4 (D) 4k,Tarrow_forwardThe probability p; of occupying an available state j is: Pj 9je-BEj Z where g; is the degeneracy of the state and 1/ẞ=kBT. Derive an expression for the temperature I in terms of the probabilities of occupation of two different states i and k.arrow_forward
- Given the internal energy U and entropy S of N weakly interacting particles in a closed system with fixed volume V. U = NkgT² (27In 2) U S = Nkg lnz + T (a) Prove the Helmholtz free energy (b) Prove the Pressure of the system is F = -NkBT ln z P = Nk Tln z) ( Tarrow_forwardConsider N non interacting particles in a gas are in thermal equilibrium and each particle can be in any one of the possible non-degenerate states of energy 0 and 28. Find the probability of each particle when temperature of the gas (T) is very much E larger than the value of (a) (b) 1 alm (d) م اما م اداarrow_forwardShow that the partition function for a simple harmonic oscillator is e-Bwħ/2 1-e-Bhw (a) Calculate the internal energy, U and show that the high-temperature result is consistent with the equipar- tition theorem result. (b) Calculate the heat capacity, Cy and show that the high-temperature result is consistent with the equipar- tition theorem result. (c) Calculate the Helmholtz function, Farrow_forward
- In quantum statistical mechanics, consider a system of independent, distinguishable particles, each of which has only two accessible states; a ground state of energy 0 and an excited state of energy ε. If the system is in equilibrium with a heat bath of temperature T, calculate A (Helmholtz free energy), E (internal energy), S (entropy), and Cv (specific heat capacity at constant volume). Does the choice of the ground-state energy equal 0 affect P (pressure), Cv, or S? How would your results change if ε0 were added to both energy values?arrow_forwardone-dimensional A one-particle, system has the potential energy function V = V₁ for 0 ≤ x ≤ 1 and V = ∞ elsewhere (where Vo is a constant). a) Use the variation function = sin() for 0 ≤ x ≤ 1 and = 0 elsewhere to estimate the ground-state energy of this system. b) Calculate the % relative error.arrow_forward2.6 (a), (b) • (a) Show that, if the kinetic energy of a particle with mass m, momentum p p²/2m, the single particle partition function can be written Z1 = V/23, where 1 = partition function for the ideal gas will then be is E = Vh2/2nmkgT is the thermal wavelength. The canonical VN ZN N! 23N • (b) Use Stirling's approximation to show that in the thermodynamic limit the Helmholtz free energy of an ideal gas is A = -NkgT In ( V +1 N23arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON