
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
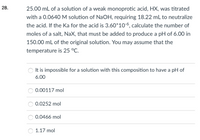
Transcribed Image Text:28.
25.00 mL of a solution of a weak monoprotic acid, HX, was titrated
with a 0.0640M solution of NaOH, requiring 18.22 mL to neutralize
the acid. If the Ka for the acid is 3.60*106, calculate the number of
moles of a salt, NaX, that must be added to produce a pH of 6.00 in
150.00 mL of the original solution. You may assume that the
temperature is 25 °C.
It is impossible for a solution with this composition to have a pH of
6.00
0.00117 mol
0.0252 mol
0.0466 mol
1.17 mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- We titrate a solution containing 10 mL of CH3COOH 0.1095 mol/L and 90 mL of distilled water, with NaOH 0.09220 mol/L, a strong base. What is, at equilibrium, the concentrations of H3O+, CH3COO-, CH3COOH and the initial pH (of the acid) before starting the titration?arrow_forward5) You need to make 250.0 mL of a buffer with a pH of 4.10, in the chemical storage room you found some formic acid, HCHO2, pK, = 3.74 and 35.9 grams of sodium formate, NACHO2. How many moles of formic acid should you use?arrow_forwardYou are titrating 90.0 mL of a weak base solution (A-) with a concentration of 0.0515 M, using an HCl solution with a concentration of 0.0662 M. Note that the Ka Value for the conjugate acid , HA, is 5.6 x 10-10 . a) What is the pH of the initial weak base solution? b) What is the pH of the solution at the equivalence point? c) What is the pH of the solution when an additional 10 mL of HCl solution is added after reaching the equivalence point?arrow_forward
- An analytical chemist is titrating 243.8 mL of a 0.7000 M solution of butanoic acid (HC3H,CO2) with a 0.3800 M solution of NaOH. The pK of butanoic acid is 4.82. Calculate the pH of the acid solution after the chemist has added 278.5 mL of the NaOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of NaOH solution added. Round your answer to 2 decimal places. PH ☐ olo Ararrow_forward1 L buffer containing 0.75 M formic acid ( HCOOH) and 0.25 M sodium formate ( HCOONa) was prepared . pka of formic acid is 3.75. Calculate the pH of the buffer when (a) when 40 mL 5 M HCI was added to the buffer (b) when 40 mL 5 M NaOH was added to the bufferarrow_forwardWhich of the following acids (listed with Ka values) and their conjugate base should be used to form a buffer with a pH of 2.34? Group of answer choices HClO2, Ka = 1.1 × 10-2 HIO, Ka = 2.3 × 10-10 HCN, Ka = 4.9 × 10-10 C6H5OH, Ka = 1.3 × 10-10 HN3, Ka = 2.5 × 10-5arrow_forward
- Suppose you have an alkaline buffer consisting of 0.20 M aqueous ammonia (NHs) and 0.10 M ammonium chloride (NH4CI). What is the pH of the solution? 5 Calculate the pH of a 0.350 M solution of potassium phenolate, KC6H5O. Ka for phenol (CH5OH) is 1.0 x 10-10. How do the concentration/volumes of the buffer affect the buffer capacity? E.g., 50.0 mL of 0.10 M acetic acid solution with 50.0 mL of 0.10 M sodium acetate solution vs. the buffer you made in the lab (25.0 mL of 0.10 M acetic acid solution with 25.0 mL of 0.10 M sodium acetate solution).arrow_forwardWhen a 18.3 mL sample of a 0.460 M aqueous hydrocyanic acid solution is titrated with a 0.330 M aqueous barium hydroxide solution, (1) What is the pH at the midpoint in the titration? (2) What is the pH at the equivalence point of the titration? (3) What is the pH after 19.1 mL of barium hydroxide have been added?arrow_forwardHow many moles of sodium hypobromite, NaBrO, should be added to 1.00 L of 0.396 M hypobromous acid, HBrO, to form a buffer solution of pH 8.95? The Ka of HOBr is 2.0 × 10–9. Express your answer in moles using at least three significant figures. Do not use scientific notation.arrow_forward
- When a 20.1 mL sample of a 0.367 M aqueous hydrocyanic acid solution is titrated with a 0.492 M aqueous barium hydroxide solution, (1) What is the pH at the midpoint in the titration? (2) What is the pH at the equivalence point of the titration? (3) What is the pH after 11.2 mL of barium hydroxide have been added?arrow_forward-2 A chemistry graduate student is given 100. mL of a 0.80M chlorous acid (HCIO,) solution. Chlorous acid is a weak acid with K,=1.1 × 10 ´. What mass of KCIO, should the student dissolve in the HCIO, solution to turn it into a buffer with pH = 1.37? You may assume that the volume of the solution doesn't change when the KC10, is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. x10arrow_forward(a) Calculate the pH of a 0.500 L buffer solution composed of 0.700 M formic acid (HCOOH, Ka = 1.77 x 10-4) and 0.500 M sodium formate (HCOONa). (b) Calculate the pH after adding 50.0 mL of a 1.00 M NaOH solution.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY