
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Problem statement is asking for volumetric flowrate*
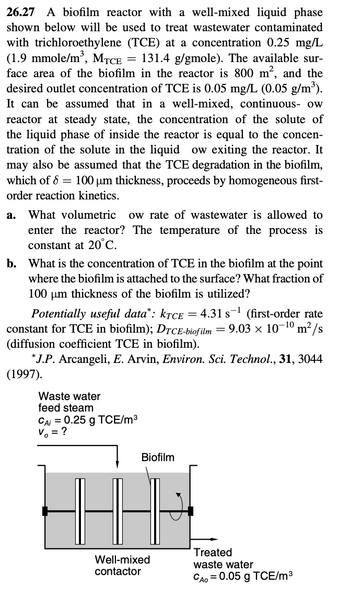
Transcribed Image Text:26.27 A biofilm reactor with a well-mixed liquid phase
shown below will be used to treat wastewater contaminated
with trichloroethylene (TCE) at a concentration 0.25 mg/L
(1.9 mmole/m³, MTCE = 131.4 g/gmole). The available sur-
face area of the biofilm in the reactor is 800 m², and the
desired outlet concentration of TCE is 0.05 mg/L (0.05 g/m³).
It can be assumed that in a well-mixed, continuous- ow
reactor at steady state, the concentration of the solute of
the liquid phase of inside the reactor is equal to the concen-
tration of the solute in the liquid ow exiting the reactor. It
may also be assumed that the TCE degradation in the biofilm,
which of 8 = 100 µm thickness, proceeds by homogeneous first-
order reaction kinetics.
a. What volumetric ow rate of wastewater is allowed to
enter the reactor? The temperature of the process is
constant at 20°C.
b. What is the concentration of TCE in the biofilm at the point
where the biofilm is attached to the surface? What fraction of
100 μm thickness of the biofilm is utilized?
Potentially useful data*: KTCE = 4.31 s ¹ (first-order rate
constant for TCE in biofilm); DTCE-biofilm = 9.03 × 10-¹0 m²/s
(diffusion coefficient TCE in biofilm).
*J.P. Arcangeli, E. Arvin, Environ. Sci. Technol., 31, 3044
(1997).
Waste water
feed steam
CAi = 0.25 g TCE/m³
V = ?
Biofilm
4-8
Well-mixed
contactor
Treated
waste water
CAO = 0.05 g TCE/m³
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Liquid is flowing through a circular pipe in a laminar phase. Now, the fluid through the pipe is replace with a more viscous fluid and passed through the pipe again with the same velocity. What will be the nature of flow of the fluid? Eddies will star to form B The flow will become turbulent Insufficient data to define The fluid will remain laminar The flow will start to be transitionalarrow_forward1. Water (p= 1000 kg m-³ and μ = 0.0011 kg m-¹ s-1) flows at 5 m s-1 over a 0.3 m long flat plat. Determine if the flow in the boundary layer is laminar over the entire length of the plat or it becomes turbulent at a downstream location. If latter is the case, determine the location of transition from laminar to turbulent. [Ans. BL flow is Laminar & turbulent, x = 0.11 m]arrow_forwardSeveral different classifications of fluid flows were outlined - match each of the class pairs listed. Viscous A. Inviscid Extrernal B. Turbulent Incompressible C. Internal Laminar D. Compressiblearrow_forward
- Q4- Water flows at a velocity of 1 m/s over a plane surface 0.6 m wide and 1 m long. Calculate the total drag force acting on the surface if the transition from streamline to turbulent flow in the boundary layer occurs when the Reynolds group Re, = 105 and Taking == 1 mN s/m² = 10-³ Ns/m².arrow_forward6.2. Air is flowing through a horizontal tube with 1.00-in inside diameter. What is the maximum average velocity at which laminar flow will be of the stable flow pattern? What is the pressure drop per unit length at this velocity?arrow_forwardlarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The