
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Answer- 828 lbf=3.68kN

Transcribed Image Text:**Problem 7.15:** The U-bend shown in Fig. 7.32 is connected to the rest of the piping system by flexible hoses. The internal diameter (ID) of the pipe is 3 inches. The fluid flowing is water, with an average velocity of 50 feet per second. The gauge pressure at point 1 is 30 psig, and at point 2 is 20 psig. What is the horizontal component of the force in the support?
(Note: Ensure students have access to Fig. 7.32 to solve this problem effectively.)
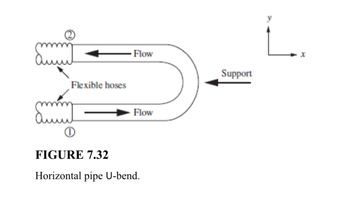
Transcribed Image Text:### FIGURE 7.32: Horizontal Pipe U-bend
The diagram illustrates a U-shaped pipe with flow dynamics, featuring flexible hoses on both ends. The direction of flow is indicated by arrows, showing fluid entering at point 1, traveling through the lower section of the U-bend, and exiting at point 2 through the upper section.
Key elements of the diagram include:
- **Flexible Hoses**: These are depicted at both ends of the U-bend, highlighting areas that allow movement and flexibility within the pipe system.
- **Flow Direction**: Arrows clearly indicate the direction of fluid flow, entering and exiting through the flexible hoses.
- **Support**: An arrow labeled as "Support" points toward the bend area, suggesting reinforcement to maintain structural integrity and balance against fluid dynamics.
- **Coordinate Axes**: The diagram features x and y axes in the top right corner, suggesting orientation and reference for analyzing flow dynamics within the horizontal plane.
The purpose of the U-bend system depicted here is likely related to managing fluid flow and accommodating pressure changes, while the flexible hoses facilitate adaptation to variations in flow conditions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- P1 P2 L3 11 12 L4 15 P3 A liquid flows through a pipe as shown above at a rate of 100 gpm. The entire piping system is 2inch inside diameter pipe Given: • The density of water is 62.4 lbm/ft3 ⚫ The specific gravity of the fluid is 0.75 • The viscosity of the fluid is 15 CP; The relative roughness of the piping is 0.0006 ⚫ Pressure gauge readings are in psig and P1 = 130 psig ⚫The piping system dimensions are as follows: L1-19ft; L2=36ft; L3-75ft; L4=10ft ; L5=18ft;arrow_forwardF1 F2 A2 P2 The following is known: Area, A1= 0.3 ft and A2= 7.6 A1- Assuming an incompressible fluid If a force F1 of 44.3 lbf / ft2 is applied at (1) then the resultant force, F2 in Ibf is equal to -- ?arrow_forwardCalculate the melting point of ice under a pressure of 10 MPa in Kelvin. Assume that the density of ice under these conditions is approximately 0.915g/cm and that of liquid water is 0.998 g/cm?.arrow_forward
- 4-24 A steady, incompressible, two-dimensional (in the xy-plane) velocity field is given by V = (0.523 – 1.88x + 3.94y)i + (-2.44 + 1.26x + 1.88y)j Calculate the acceleration at the point (x, y) = (-1,55, 2.07).arrow_forwardP1arrow_forwardPlease get this right! I'll upvote super fast if you do!!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The