
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
2,5-Dimethylfuran (DMF), which can be made from cellulosic biomass, has been suggested as a possible liquid transportation fuel. DMF has 40% greater energy density by mass than ethanol based on HHV. DMF is C6H8O. Compare the LHV of dimethylfuran and ethanol on a weight and volume basis. Use the data in Table 2.4 for ethanol. The specific gravity of DMF is 0.9
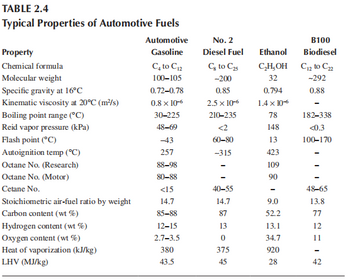
Transcribed Image Text:TABLE 2.4
Typical Properties of Automotive Fuels
Property
Chemical formula
Molecular weight
Specific gravity at 16°C
Kinematic viscosity at 20°C (m²/s)
Boiling point range (°C)
Reid vapor pressure (kPa)
Flash point (°C)
Autoignition temp (°C)
Octane No. (Research)
Octane No. (Motor)
Cetane No.
Stoichiometric air-fuel ratio by weight
Carbon content (wt%)
Hydrogen content (wt%)
Oxygen content (wt%)
Heat of vaporization (kJ/kg)
LHV (MJ/kg)
Automotive
Gasoline
C4 to C₁2
100-105
0.72-0.78
0.8 x 10-6
30-225
48-69
-43
257
88-98
80-88
<15
14.7
85-88
12-15
2.7-3.5
380
43.5
No. 2
Diesel Fuel
Cg to C₂5
-200
0.85
2.5 x 10-6
210-235
<2
60-80
-315
40-55
14.7
87
13
0
375
45
Ethanol
C₂H, OH
32
0.794
1.4 x 10-
78
148
13
423
109
90
9.0
52.2
13.1
34.7
920
28
B100
Biodiesel
C₁2 to C₂2
-292
0.88
182-338
<0.3
100-170
48-65
13.8
77
12
11
L
42
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- 2. Carbon nanotubes (CNT) are among the most versatile building blocks in nanotechnology. These unique pure carbon materials resemble rolled-up sheets of graphite with diameters of several nanometers and lengths up to several micrometers. They are stronger than steel, have higher thermal conductivities than most known materials, and have electrical conductivities like that of copper but with higher current- carrying capacity. Molecular transistors and biosensors are among their many applications. While most carbon nanotube research has been based on laboratory-scale synthesis, commercial applications involve large industrial-scale processes. In one such process, carbon monoxide saturated with an organo-metallic compound (iron penta- carbonyl) is decomposed at high temperature and pressure to form CNT, amorphous carbon, and CO2. Each "molecule" of CNT contains roughly 3000 carbon atoms. The reactions by which such molecules are formed are: Decomposition of Fe(CO)s to form iron, which…arrow_forwardCan you reword this for me to have a better understanding: Plastic items like bottles and bags are made from polyethylene. Ethylene Oxide / Ethylene Glycol – becomes polyester for textile, and antifreeze for airplane engines and wings. Ethylene Dichloride – this, in turn, becomes a vinyl product used in PVC (polyvinyl chloride) pipes, siding, medical devices, and clothing. Styrene – synthetic rubber found in tires, as well as foam insulation.”arrow_forward15.00 ml of a 0.3400 m manganese(iii) acetate solution is mixed with 25.00 ml of a 0.2311 m ammonium carbonate solution. Calculate the number of grams of solid precipitate produced. Calculate the molarity of all species in solution after the reaction has taken place.arrow_forward
- For a stoichiometric amount of methane-air mixture calculate the mass fractions and stoichiometrically weighted mass fractions (with the fuel being the reference species and YF, B = 1) of O₂ and CH4 before reaction, and of CO₂ and H₂O after complete reaction without dissociation. What can you say about Ỹ; and the small value of YCH4 relative to Yo₂ in terms of the suitability of hydrocarbons as transportation fuels?arrow_forward1. Water Quality Analysis - A public utility announces the monthly average concentrations of several ionic species in the drinking water. The list is given below (atomic weights are given in parentheses): Calcium (40) = 8.6 mg/L Magnesium (24)=0.77 mg/L Potassium (39) = 0.64 mg/L Sodium (23) = 3.7 mg/L a) What is the ratio of [HCO3]/[CO3²] in this water Chloride (35.5) -3.0 mg/L Fluoride (19)- 0.84 mg/L Sulfate (S-32; O=16)= 3.4 mg/L pH=8.46arrow_forwardUsing RCRA procedures, determine if the following are hazardous wastes. State the reason why, or why not it is hazardous. If it is hazardous, state the category number. Assume the industry producing the waste is a RCRA hazardous waste generator. 1. A drum of sulfuric acid at pH 1.2. A drum of waste trichloroethylene (22% by volume).arrow_forward
- We are tasked to do iteration in mass and energy balances, wherein we will look for the final temperature. When there are two components, like CO and CO2, do you add their enthalpy out (i.e. 3.376 + 0.557x10-3T - 0.031x105T-2 for CO and 5.457 + 1.045x10-3T - 1.157x105T-2 for CO2) to use for the calculation of Cp, mean? If yes, what would be the value of Cp, mean?arrow_forwardQ1): Tw~362 K; Tn~342 Karrow_forwardA catalyst was created to convert C3H8O into C3H6O through a gas-phase reaction at 450 K and 0.75 bar: C3H8O = C3H6O + H2 The feed is C3H8O and N2 (an inert) at a 1:4 mole ratio. At thermodynamic equilibrium, determine percent conversion of C3H8O. Assuming the reaction proceeds to equilibrium, how many kilograms of C3H8O would be needed to produce 5 kilograms of C3H6O?arrow_forward
- (Willing to repost the other questions, please help) A continuous steady-state distillation column with a total condenser and a partial reboiler isseparating 1-butanol from cyclohexanol at 1 atm. 1000 kmol/h of the said binary mixtureconsisting of 45 mol% 1-butanol is being fed to the column. It is desired to have a distillate productthat is 95 mol% 1-butanol and a bottoms product that is 2 mol% 1-butanol. Assume CMO. Thereflux ratio and the boil-up ratio are 1.30 and 1.80, respectively.a) Perform mass balances to plot the enriching section, stripping section, and the feed line.b) What type of feed is used in the column?c) Find number of ideal stages for desired separation and the optimal feed tray location.d) Determine the concentration of the mixture at each stage.e) Calculate the flowrates of the distillate and bottoms, as well as the reflux and boil-off.f) Find the minimum number of stages required to achieve the desired separation.g) Find the minimum reflux ratio. If needed…arrow_forwardQUESTION 6 The equilibrium constant for the reaction is 3.0x104 at 310 K. At equilibrium, the partial pressure of H2 S(g) is 0.370 atm. Calculate the concentration, expressed in units of mM (millimolar) of ammonia gas? Enter your answer in deimal notation and provide 3 significant figures. For example, enter 0.2531 as 0.253arrow_forwardUsing the provided ternary phase diagram 1) Determine the EXACT composition of point a2. 2) Determine the maximum amount of (in moles) of pure E that can be added to a 10 mol mixture at point a2 to maintain a two-phase system?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The