
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
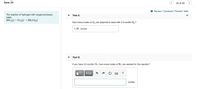
Transcribed Image Text:Item 24
24 of 25
I Review I Constants I Periodic Table
The reaction of hydrogen with oxygen produces
Part A
water.
2H2 (g) + O2 (g) → 2H2O(g)
How many moles of O2 are required to react with 2.0 moles H2?
1.95 moles
Part B
If you have 5.0 moles O2, how many moles of H2 are needed for the reaction?
moles
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- urces xGveUp Hint Combining 0.342 mol Fe,O, with excess carbon produced 11.2 g Fe. Fe,0, + 3C – 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? mol theoretical yield: What is the percent yield? % percent yield: 63 F Sunny A ôarrow_forward#3arrow_forwardE-85 is an alternative fuel for automobiles and light trucks that consists of 85.0% (by volume) ethanol (C2H5OH), and 15.0% gasoline. When ethanol burns completely it produces CO2 and H2O. The balanced equation for the burning of ethanol isC2H5OH+3O2-->2CO2+3H2OThe density of ethanol is 0.790 g/mL. How many moles of carbon dioxide are produced by the complete combustion of the quantity of ethanol in 8.00 gallons of E-85 fuel? _mol CO2arrow_forward
- If 6.3 kg of CO₂ are produced during a combustion reaction, how many molecules of CO₂ would be produced? -don't prematurely roundarrow_forwardItem 15 15 of 25 I Review I Constants I Periodic Table Carbon disulfide and carbon monoxide are produced when carbon is heated with sulfur dioxide. Part A 5C(s) + 2SO2 (g) → CS2(1) + 4CO(g) How many moles of C are needed to react with 0.460 mole SO2? Express your answer using three significant figures. ? moles Part B How many moles of CO are produced when 2.1 moles C reacts? Express your answer using two significant figures. Ην ΑΣφ molesarrow_forwardCombining 0.229 mol Fe2O30.229 mol Fe2O3 with excess carbon produced 13.0 g Fe.13.0 g Fe. Fe2O3+3C⟶2Fe+3COFe2O3+3C⟶2Fe+3CO What is the actual yield of iron in moles? actual yield: What is the theoretical yield of iron in moles? theoretical yield: What is the percent yield? percent yield: %arrow_forward
- If 2.80 moles of H2 and 1.55 moles of O2 react how many moles of H20 can be produced in the reaction below? 2 H2(g) + O2(g) → 2 H2O(g) | mol 1 4 6. C 7 8 9. +/- х 100 LOarrow_forwardIn the following chemical reaction how many moles of Cl2 will react with 2 moles of P4? P4 + 6Cl2 ----> 4PCl3arrow_forwardNeed help on botharrow_forward
- What is that mass of glucose (180.18g/mol) produced from 2.20g of CO2arrow_forwardIf 94.1g Cr are reacted how many grams Cr2O3 will be formed? Pertaining to 4Cr + 3O2 > 2Cr2O3arrow_forwardConsider the following reaction: P₂O(s) Pals) 30(9) What mass of P4 will be produced from 12.0 g of P₂O.? = (Molar massses: P406-219.9 g/mol, P4 - 123.9 g/mol) 6.76 g 21.3 g 0.563 g 1.77 g Consider the reaction below: BaCl2 + Na2SO4- BaSO4 + 2 NaCl (balanced) What is the theoretical yield of barium sulfate, BaSO4, that can be produced by the reaction of 2.84 g of Na₂SO with 5.00 g of BaCl₂? molar mass of BaCl2 = 208.33 g/mol, molar mass of BaSO, 233.38 g/mol, 2.38 g 4.67 g 5.60 g 1.73 g 0000 molar mass of Na2SO4 = 142.04 g/mol, molar mass of NaCl = 58.44 g/molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY