
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
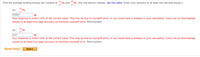
Transcribed Image Text:24
Mg and
12
85
Find the average binding energy per nucleon of
Rb. (For the atomic masses, see this table. Enter your answers to at least two decimal places.)
37
24
(a)
Mg
12
8.0017
Your response is within 10% of the correct value. This may be due to roundoff error, or you could have a mistake in your calculation. Carry out all intermediate
results to at least four-digit accuracy to minimize roundoff error. MeV/nucleon
85
Rb
37
(b)
8.47
Your response is within 10% of the correct value. This may be due to roundoff error, or you could have a mistake in your calculation. Carry out all intermediate
results to at least four-digit accuracy to minimize roundoff error. MeV/nucleon
Need Help?
Read It
Expert Solution
arrow_forward
Step 1
This question is from chapter Atomic Structure where we are talking about the binding energy.
As we know mass of nucleus is always less than the sum of the masses of the protons and neutrons that make up the nucleus.
Tha difference is nothing but the binding energy.
Binding energy per nucleon is given by binding energy divided by the total number of nucleons(the mass number of that element).
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the binding energy of 11C. The atomic mass of 11C is 1.82850 \times 10-26 kg. x 10 Jarrow_forwardSome Radioactive Isotopes Useful in Medical Imaging Mode of Isotope Decay Half-life Use in Medical Imaging B+, Y B+, Y Carbon-11 20.3 m Brain scan to trace glucose metabolism Brain scan to trace glucose metabolism Detect eye tumors Diagnose albinism, image the spleen and gastrointestinal tract Bone marrow function, diagnose anemias Whole-body scan for tumors Pancreas scan F Fluorine-18 109 m 14.3 d Phosphorus-32 Chromium-51 Iron-59 Gallium-67 Selenium-75 Krypton-81m Strontium-81 E.C., Y B, Y E.C., Y E.C., Y Cr Fe SGa 27.7 d 44.5 d 78.3 h 118 d 13.3 s Lung ventilation scan Scan for bone diseases, including cancer Brain, liver, kidney, bone scans; diagnosis of damaged heart muscle Diagnosis of thyroid malfunction Kidney scan Heart scan and exercise stress test Sr 22.2 m 38 99m Te 43 Technetium-99m 6.01 h Iodine-131 В, у E.C., Y E.C., Y 8.04 d Mercury-197 Thallium-201 64.1 h 3.05 darrow_forwardvalue needs to be unroundedarrow_forward
- Calculate the binding energy per atom for 23 Na which has a mass of 22.9898 amu. The masses of single 11 protons, neutrons and electrons are 1.0073, 1.0087 and 0.00055 amu respectively. Report your answer in MeV to one place after the decimal. Add your answer Integer, decimal, or E notation allowedarrow_forwardVISUAL IZATION Modes of Radioactivve Decay 140 130 120 110 100 90 80 70 60 50 - Stable - Radioactive 40 30 20 10 10 20 30 40 50 60 70 80 90 100 Number of protons (Z) Now consider positron emission. Now consider electron capture. "E8+R "E+ge +",R n-1 In which direction would a nucleus move on this chart if it In which direction would a nucleus move on this chart if it were to emit a positron? were to occur? up to the left O up to the left O up to the right up to the right down to the left down to the left down to the right down to the right For what types of unstable nuclei (compared with those on For what types of unstable nuclei (compared with those on the island of stability) will this form of radioactivity be mos the island of stability) will this form of radioactivity be most likely to occur? likely to occur? those with excess neutrons O those with excess neutrons O those with not enouah neutrons O those with not enough neutrons N) suognau jo aqunNarrow_forward2. Complete the following nuclear equation and identify X in each case: 26 a.) Mg + p - a + X b.) co + {H 60co + X 27 27 c.) 35u 94Kr + 36 139 56 Ba + 3X 92 53C 24 4 a + X d.) Cr e.) 280 20F + X A sample containing Br-82 was found to uore nreser urcarrow_forward
- 7arrow_forward12. Calculate the nuclear binding energy per one nucleon for the Be-8 isotope (eight nucleons). Here are some helpful data: {B.E = Am .c2 c=3.00 x 108 m/s and I J= kg.m2/s2} 1.32931 x 10-23 g 1.67262158 × 10–24 g 1.67492716 x 10–24 g Actual mass of Be- 8 proton mass neutron mass a) 1.2035 x 10–6 J/nucleon c) 1.092263 x 10–12 J/nucleon b) 1.092263 x 10–9 J/nucleon d) 8.7381 x 10–12 J/nucleonarrow_forwardWhat is the binding energy per nucleon for Kr-93, which has a mass of 92.93113 amu. please show work:) It helps me better understand the process of working irbourarrow_forward
- What fraction of the decay energy is this, noting that the decay mode is π0 → ϒ+ϒ (so that all the π0 mass is destroyed)?arrow_forward2 Calculate the binding energy (J/nucleon) for Uranium-235 (molar mass= Mass of proton = 1.67262192369E-27 kg Mass of a neutron 1.67492749804E-27 kg Mass of an electron = 9.1093837015E-31 kg Speed of light = 299792458 m/s NA = 6.022E23 particles / mol = Enter a number - no units. 235.04393 g/mol).arrow_forwardCalculate the binding energy per nucleon of 56Fe. Write answer in MeVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY