
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
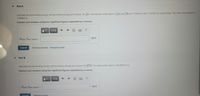
Transcribed Image Text:Part A
Calculate the total binding energy and the binding energy per nucleon, for Li. The masses of the atoms of Li and H are 7.016004 u and 1.007825 u. respectively The mass of a neutron is
1.008665 u.
Express your answers using four significant figures separated by a comma.
V AEd
Erotal, Eper nucleon =
MeV
Submit
Previous Answers Request Answer
Part B
Calculate the total binding energy, and the binding energy per nucleon, for 105 Pt The mass of the atom is 194 964774 u
Express your answers using four significant figures separated by a comma.
Vo AE
?
Eutal, Eper nucleon=
MeV
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardCalculate the mass defect for the neon-21 nucleus. The mass of neon-21 is 20.993847 u. Please help, I'm confused because my calculations don't check outarrow_forward3 eq req req 2req 2req 3 E D Q C Write a balanced nuclear equation for the decay of bismuth-214 to polonium-214. Submit Answer $ 4 R FL V 07 20 % 5 T [Review Topics] [References] Use the References to access important values if needed for this question. Retry Entire Group 9 more group attempts remaining G Cengage Learning | Cengage Technical Support B 6 5) + T MacBook Pro Y H & 7 N U J * 00 8 + I M ( 9 K ; + "1 { [ 21 Save and Exit ? 11arrow_forward
- Item 14 Part A The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the binding energy per nucleon (in J) of a Coancleus ? (The mass of a cobalt-62 nucleus is 81.9341 amu.) O 8.46x10-J O 4.15x10-9 J O 1.40x10-34 J O 8.20x10-11 J 8.46x10-11 J Submit Request Answer Provide Feedbackarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardCalculate nuclear binding energy per nucleon (in MeV) for 239 U, which has a mass of 239.05429 amu Express your answer to four significant figures.arrow_forward
- MAIN IDEA: Nuclear fusion reactions are exothermic and produce significantly more energy per mass of fuel than fission reactions. 5. Look at Figure 1 showing the relationship between binding energy and mass number. Summarize how the slope of the line for mass numbers 80 and higher compares to the slope of the line for mass numbers 20 and lower. Summarize how this relates to the relative amount of energy produced by fission reactions and fusion reactions. BAB Binding Energy v. Mass Number Binding energy per nucleon (MeV/nucleon) 10 9 8 7 5 3 2 1 0 0 40 iron-56 - region of maximum stability 80 120 160 Mass number, A (number of nucleons) 200 240arrow_forwardWhat is the binding energy per nucleon for Kr-93, which has a mass of 92.93113 amu. please show work:) It helps me better understand the process of working irbourarrow_forward1 attempts left Be sure to answer all parts. Name the unidentified species and write the transmutation process in shorthand notation for the gamma irradiation of a nuclide yielding a proton, a neutron, and 2⁹Si. Unidentified species: 31P Transmutation process: 31P+y-1H+In+29Si Check my work (Omit all atomic numbers for the nuclides.)arrow_forward
- text f5 A % 5 T Calibri 1 16 ¹ 4 1.8 6 - Y 13. The following equation models the beta decay of krypton-89 into rubidium-89. 89 Kr 36 f7 (1) 18 2 & Explain why this reaction is not an example of alpha decay. 7 89 14. Gamma radiation is used to treat certain types of tumors deep in the brain that cannot be accessed with surgery. Suggest a possible reason to explain why gamma radiation is preferred over beta decay or alpha decay for this treatment. fg B U Rb + e - 3 V IUA O fg 8 f10 insert 9 O f11 ☆ O 5 P F12 ▼ $ ✓= prt scr + H 7 G 63°F Cloudy ^ E ] CZ1 delete backspacearrow_forwardWhen the nuclide cesium-129 decays by electron capture: The name of the product nuclide is The symbol for the product nuclide is Submit Answer Retry Entire Group 9 more group attempts remaining s [Review Topics] [References] Use the References to access important values if needed for this question. A Previous ?^arrow_forwardq req 2req بھر میں 3 E D Q C Complete the following nuclear bombardment equation by filling in the nuclear symbol for the missing species. 235U+ on Submit Answer $ 4 R F V % 5 Retry Entire Group 9 more group attempts remaining T [Review Topics] [References] Use the References to access important values if needed for this question. G Cengage Learning | Cengage Technical Support 6 B MacBook Pro Y H & 7 N E U J * 00 8 I M - 0 + Sr+ 2n 9 K e O < B X -O L ** P A - V Previous Email Instructor P { + 11 [ Next Save and Exit 11 ? 1 1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY