
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
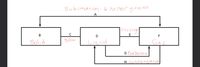
Transcribed Image Text:Sublimation: 670 Pergram
A
C
E
D
F
8Ocal
Solid
Gas
Liquid
G eva Poratia
H Condensatia

Transcribed Image Text:20. Which state of matter exhibits the highest degree of molecular motion?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the physical state of tungsten (solid, liquid, or gas) at a temperature of 5993K if the melting point of tungsten is 6182oF and the boiling point is 10100oF?arrow_forward3)arrow_forwardCondensation occurs when liquid phase of a substance is converted to its gaseous phase true/falsearrow_forward
- Chlorine trifluoride (ClF3) melts at -76.3 °C and boils at 11.8 °C at a constant pressure of 1 atm. What state of matter must a sample of chlorine trifluoride be in at 20°C and 1 atm?arrow_forwardIt is snowing outside! This process is described by which of the following? Ocondensation O evaporation O sublimation O deposition Freezingarrow_forwardX Bb DO: MLM HW 2.5- Blackbr X ck Mind Tap - Cengage Learnir X ml?deploymentid=5735112480241329813180832311&elSBN=9781305862883&id=1774598910&snapshot..... The following information is given for silver at 1 atm: boiling point = 2212 °C melting point = 961 °C specific heat solid = 0.238 J/g°C specific heat liquid = 0.285 J/g°C Submit Answer ase Change Energetics: This is group attempt 1 of 10 Use the References to access important values if needed for this question. References AHvap(2212 °C) = 254 kJ/mol AHfus (961 °C) = 12.0 kJ/mol What is AH in kJ for the process of freezing a 29.0 g sample of liquid silver at its normal melting point of 961 °C. MacBook Air G To analyze the reaction, firs X kj Autosaved at 9:15 PM Bbarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning