
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
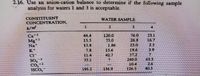
Transcribed Image Text:2.16. Use an anion-cation balance to detcrmine if the fellowing sample
analysis for waters 1 and 3 is acceptable.
CONSTITUENT
CONCENTRATION,
WATER SAMPLE
1.
76.0
26.8
15.5
11.8
7.8
11.4
33.1
120.0
75.0
1.86
156
42.7
19.6
37.2
240.0
10.4
126.5
16.7
23
39
SO,
Co,-2
11CO,
63.5
26
303
195.2
156 9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- "Rubbing alcohol" is a mixture that is 70.% isopropyl alcohol (C3H8O) in water. The mixture has a density of 0.79 g/mL at 20°C. What is the concentration of the isopropyl alcohol in molarity? The molar mass of isopropyl alcohol is 60.11 g/mol. a. 11.6 M b. 13.1 M c. 9.2 M d. 30.7 Marrow_forward2.000 g TUMS tablet (CaCO3 and Binder) was analyzed in a lab. 11.42 mL of 0.871 M HCI was needed to dissolve the CaCO3 (active ingredient) of TUMS Tablet. Find the mass and % by mass of CaCO3 in TUMS Tablets. ( 459.00 mg O 75% 50% 605.00 mg 30% O 25% O 400.00 mg O 500,00 mgarrow_forwardWhat is the mass of H2SO4 (molar mass 98 g/mol) in 400. mL of 0.100 M solution? O 3.92 g O 4.90 g 2.45 g 9.80 g MacBook Air 吕口 F3 F8 F9 F10 F4 F5 F6 F7 F1 2$ % & 2 4. 7 9- * CO 6 %#3arrow_forward
- 1. Calculate the percent yield of X when solvent D is used. Answer is 4 significant figures with proper units.arrow_forwardEqual volumes of Solutions 2 and 3 were mixed in the lab. Suggest a measurement that could be made in the lab to provide evidence that a chemical reaction occurred when Solutions 2 and 3 were mixed.arrow_forwardPercent by volume (% v/v) of a substance is a term that represents the concentration of the substance. It refers to mL of the substance per 100 mL of its solution in water. A bleach solution bought from a Walmart store contains 5% sodium hypochlorite by volume. How many mL of the bleach solution should be taken to make 2.5 L of 0.1% sodium hypochlorite solution?arrow_forward
- . Prepare 50 mL of an aqueous solution of 2.5% (w/v) NaCl (sodium chloride, salt).Determine how many grams of sodium chloride are required. Show all steps for this calculation, using dimensional analysis with units clearly identified.2. Prepare this aqueous solution, using a volumetric flask for the precise final volume measurement. It is not a problem to use tap water.3. Clearly write the procedure (step by step) for making this solutionarrow_forwardThe distinguishing characteristic of all electrolyte solutions is that they _____. contain molecule conduct electricity react with other solutions always contain acids conduct heatarrow_forwardWhen 17.4 ml of a solute is dissolved into 25.0 ml of water, the volume of a solution becomes 28.2 ml. What is the v/v concentration of the solution? 1.38.3% 2.61.7% 3.88.7% 4.30.4% 5.69.6%arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY