
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
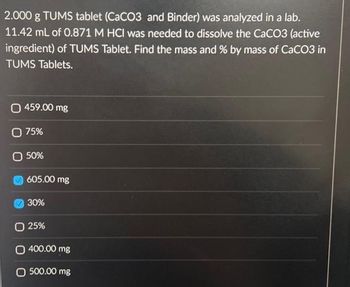
Transcribed Image Text:2.000 g TUMS tablet (CaCO3 and Binder) was analyzed in a lab.
11.42 mL of 0.871 M HCI was needed to dissolve the CaCO3 (active
ingredient) of TUMS Tablet. Find the mass and % by mass of CaCO3 in
TUMS Tablets.
( 459.00 mg
O 75%
50%
605.00 mg
30%
O 25%
O 400.00 mg
O 500,00 mg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If you weighed 0.9867 g of Zn pellets and prepared a standard solution in a 250.0 mL volumetric flask, What is the concentration of this solution? The molecular weight of the zinc pellets is 65.39 g/mol. 0.01509 M 0.0604 M 0.06036 M 6.04e-5 M 0.003772 M 258.1 M 6.036e-5 Marrow_forwardHow many mL of water should be added to 50.0 mL of a 15.0 M H2SO4 solution to give a final concentration of 0.300 M? A) 950 mL B) 1000 mL C) 2450 mL D) 2500 mLarrow_forwards (Ba²+) i A sample of 0.6760 g of an unknown compound containing barium ions is dissolved in water and treated with an excess of Na₂SO4. If the mass of the BaSO4 precipitate formed is 0.7548 g, what is the percent by mass of Ba in the original unknown compound? Round your answer to 4 significant digits. % Ba xarrow_forward
- G TuT https://app.101edu.co If 24.6 g of KBr (MM = 119.00 g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of KBr in the resulting solution? Type here to search F3 K $ Et F5 % n F6 Question 28 of 32 F7 99+ DELL F8 L g & F10arrow_forwardCould I please have help with the following problems.arrow_forwardHow many mL of 0.40 M CuSo4*5H2O are needed to make 10.0 mL of 0.080 M CuSo4*5H2O?arrow_forward
- About 40.00 mL of Na2S2O3 are needed to react with 4.0 x 10^-3 moles of I2. 2S2O3^-2 + I2 -> S4O6^-2 + 2I^-1 Calculate the molarity of the Na2S2O3 solutionarrow_forwardA student weighs out a 3.06g sample of MgBr2, transfers it to a 135mL volumetric flask, adds enough water to dissolve it then adds water to the 125mL tick mark. What is the molarity of magnesium bromide in the resulting solution? Molarity = _____ Marrow_forward8. A 200 mL dilute solution of anhydrous CuSO4 with molarity of 0.28 M was made by adding 56 mL of a stock solution, then filling to the 200 mL mark with water. What's the concentration of the original stock solution? M₁ V₁ = M₂ V₂arrow_forward
- 11. If 56.1 grams of NaCl are dissolved in 44.0 mL of Sulfuric acid, H2SO4 Sulfuric acid has a density of 1.84 grams per mL. Calculate the following concentration measures. a. Weight/volume percent b. Weight/weight percent c. Molarityarrow_forwardI'm a bit confused with this one. thank youarrow_forward6. Calculate the percent of BaO in 29.0 g of a mixture of BaO and CaO which just reacts with 100.8 mL of 6.00 M HCI. yields BaO + 2HC BaCl₂ + H₂O 65.5 % 55.6 % O 75.6 % 86.5 % CaO + 2HCl yields CaCl₂ + H₂Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY