
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
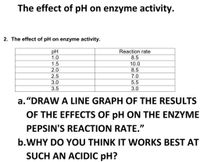
Transcribed Image Text:The effect of pH on enzyme activity.
2. The effect of pH on enzyme activity.
pH
1.0
Reaction rate
8.5
1.5
10.0
2.0
8.5
2.5
3.0
7.0
5.5
3.5
3.0
a. “DRAW A LINE GRAPH OF THE RESULTS
OF THE EFFECTS OF pH ON THE ENZYME
PEPSIN'S REACTION RATE."
b.WHY DO YOU THINK IT WORKS BEST AT
SUCH AN ACIDIC pH?
5O50 5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- 5. By using Excel or GoogleSheets. graph the Lineweaver-Burk plots for the behavior of an enzyme for which the following experimental data are available. What are the Km and Kwax values for the inhibited and uninhibited reactions? Is the inhibitor competitive or noncompetitive? [S] (mM) V, No Inhibitor (mmol min-) V, Inhibitor Present (mmol min-') 1 × 10-4 5 × 10-4 1.5 x 10-3 2.5 x 10-3 5 x 10-3 0.026 0.010 0.092 0.136 0.040 0.086 0.150 0.120 0.165 0.142arrow_forward2. Enzyme-catalyzed reactions. Answer the following with true or false. If false, explain why. (a) The initial rate of an enzyme-catalyzed reaction is independent of substrate concentration. (b) At saturating levels of substrate, the rate of an enzyme-catalyzed reaction is proportional to the enzyme concentration. (c) The Michaelis constant Km equals the substrate concentration at which velocity (v) = Vmax/2. (d) The Km for a regulatory enzyme varies with enzyme concentration. (e) If enough substrate is added, the normal Vmax of an enzyme-catalyzed reaction can be attained even in the presence of a noncompetitive inhibitor. (f) The Km of some enzymes may be altered by the presence of metabolites structurally unrelated to the substrate. (g) The rate of an enzyme-catalyzed reaction in the presence of a rate-limiting concentration of substrate decreases with time. (h) The sigmoidal shape of the v versus [S] curve for some regulatory enzymes indicates that affinity of the enzyme for the…arrow_forwardLabel the following equation. The parentheses represent the choices for each label (A-D). A. (Water, glucose, Carbon dioxide, Oxygen). B. (Water, glucose, Carbon dioxide, Oxygen) C. (Water, glucose, Carbon dioxide, Oxygen) D. (Water, glucose, Carbon dioxide, Oxygen)arrow_forward
- 15) The graph at right shows the results of reaction rate vs. substrate concentration for a Michaelis-Menten type enzyme 16) Th a. True b. False* reaction rate substrate concentrationarrow_forwardV 23. The graph below is a graph of Vmax (a) Label the graph clearly with both the Vmax and the Km. Estimate the Km from this graph giving the correct units. v/Vmax 1.0 0.5 0.0 0.0 vs [S] for an enzyme. Enzyme Activity vs [Substrate] :0.2 0.4 Substrate (HM): 0.6 (b) If the Vmax = 25 mmoles per minute per µmole of enzyme calculate the Keat and the specificity constant."arrow_forward4. a. Calculate the KM (Michaelis constant) and the vmax (the maximum initial rate) for both substrates (sphingosine and ATP). Show your work, and be careful about units. b. threo-dihydrosphingosine, a stereoisomer of sphingosine, is an inhibitor of sphingosine kinase. What kind of inhibitor (competitive, uncompetitive, noncompetitive) is threo-dihydrosphingosine? Citing information from the Lineweaver-Burke plots, explain how you can tell.arrow_forward
- Select all FALSE statements about allosteric enzymes. a. They interconvert between a more active form and a less active form. b. They tend to have a hyperbolic curve of ?0 vs. [S]. c.They conform to Michaelis–Menten kinetics. d. They are generally small single subunit proteins. e. They may have binding sites for regulatory molecules that are separate from active sites.arrow_forward1. The concentration of substrate X is high. What happens to the rate of the enzyme-catalyzed reaction if the concentration of substrate X is reduced? Explain. 2. An enzyme has an optimum pH of 7.2. What is most likely to happen to the activity of the enzyme if the pH drops to 6.2? Explainarrow_forward1a.Sketch a graph that shows an enzyme that functions at an optional pH of b. which organ of the body does it likely to work at? 2. On the same graph from la, sketch another line/curve that shows what happen to the enzyme activity (in #1a) if a constant [ ] of inhibitors is added.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education