
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
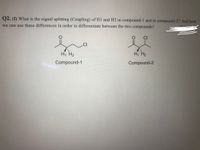
Transcribed Image Text:Q2. (I) What is the signal splitting (Coupling) of H1 and H2 in compound-1 and in compound-2? And how
we can use these differences in order to differentiate between the two compounds?
CI
.CI
H, H2
H, H2
Compound-1
Compound-2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following would not theoretically be seen in the splitting patterns for the 1Hb NMR signals for the following molecule if Jab < Jbc . CH; „Hb H;aC `CH2c-Br A. type b splits type a into a doublet B. type b splits type c into a doublet O C. type a splits type b into a septet then type c splits those into triplets O D. type c splits type b into a triplet then type a splits those into septetsarrow_forwardA STK2083_Molecular_Spectroscopy_Group Assignment.pdf - Adobe Acrobat Reader DC File Edit View Sign Window Help Home Tools STK2083_Molecular. x Sign In 1 /1 119% a. Which of the compounds below best matches the following 'H NMR spectrum? Integration values are indicated next to their corresponding signal. 2. Search 'Signature' Convert PDF но Edit PDF A B Comment OH HO Combine Files D E EI Organize Pages 24 16 2 Redact A Compress PDF 16 U Protect 8 D Fill & Sign Create, edit and sign PDF forms & agreements PPM b. Briefly explain your answer 2 (a) based on number of signals, position of signals and the integration of the signals. Start Free Trialarrow_forwardSPECTROSCOPY - Attached are the IRs for Caffeine and Adenine (Molecular structures on Lab Manual p. 34). Please (a) NAME each compound at the bottom of each spectrum box. (b) Label the major peaks with the proper functional group for each spectrum. 100- Transmittance (%) Transmittance (%) 50- 4000 100 50 3303 34 1673 10 3123 20 1605 4 2982 42 1508 74 2800 43 1471 72 2710 50 1463 74 2892 49 1451 65 2601 68 1419 36 0 4000 3000 2925 4 2854 14 1699 10 1859 10 1699 49 3110 64 1649 31 2954 11 1457 21 1431 37 1404 56 1378 49 1360 49 1326 68 1368 60 1334 43 1309 27 1263 46 1156 81 1126 88 1023 72 3000 1286 63 1240 36 1213 80 1189 62 1072 77 1026 57 974 68 2000 1500 Wavenumber (cm-¹) 940 38 913 55 875 77 849 72 797 64 792 84 724 49 643 49 622 60 543 57 2000 1500 Wavenumber (cm-¹) 81 927 873 77 851 74 769 60 746 23 700 84 646 84 wwwm 638 86 610 57 482 49 1000 -END- 500 1000 500 38arrow_forward
- 16) The splitting of a signal in a ¹H NMR spectrum is determined by A. The number of neighboring B. The electronic environment of the C. Number of enantiotopic D. Number of equivalent E. Number of non-equivalent protons.arrow_forward5. Use the 1H-NMR data to determine the structure of the compounds listed below. Indicate which hydrogens are responsible for each signal. a. C2H4C|z: singlet at s 3.7 ppm b. C8H10: § 1.2 ppm (triplet, 3H) § 2.6 ppm (quartet, 2H) § 7.1 ppm (broad singlet, 5H)arrow_forwardThe methyl protons in acetaldehyde appear at a shift of 2.20 ppm, while the aldehydic proton appears at 9.80 ppm. In a 60 MHz NMR with an external field of 1.5 T, what is the magnetic field experienced by each type of proton? Explain why the presence of 3 protons on the adjacent carbon split the aldehydic proton peak into a quartet.arrow_forward
- Give answer all questions with explanation pleasearrow_forwardShown below are carbon NMR spectra for various isomers of C5H11Br. Assign structures for the compound that would produce each spectrum.arrow_forwardImine compounds usually show stretching bands in the IR in the range of 1690-1640 cm-1 and aromatic amines show a C-N stretch at 1340-1265 cm1. A related compound, N-benzylideneaniline, does not show tautomerism and has a C...N stretch at 1678 cm-1 while H2SB-1 shows a C..N stretch of 1590 cm-1. Given this information, does H2SB-1 show some C..N single bond character? но ono N' OH H2SB-1: 1590 cm-1 N-Benzylideneaniline: 1678 cm1arrow_forward
- 14) The intensity of a signal in a ¹H NMR spectrum is determined by A. The number of neighboring C. Number of enantiotopic B. The electronic environment of D. Number of equivalent E. Number of non-equivalent protons.arrow_forwardQ.5. What is the most likely molecular formula of a compound with m/z of 101 for its molecular ion, and m/z of 30 for its base peak in its EI mass spectrum. Write two isomers of this compound (more isomers are possible).arrow_forwardQuestion 9 of 18 Match the peaks in this spectrum with the carbons on the structure below. A) : 178.1 рpm II: 68.8 ppm III: 22.5 ppm IV: 27.9 ppm B) I: 178.1 ppm II: 22.5 ppm I: 68.8 рpm IV: 27.9 ppm 160 C) I: 178.1 ppm O. II: 27.9 ppm III IV II: 22.5 ppm IV: 68.8 ppm D) I: 68.8 ppm II: 27.9 ppm II: 22.5 ppm IV: 178.1 ppmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
